
QUANTITATIVE BIOCHEMICAL ASSAY ON A SURFACE
MICROFLUIDIC DEVICE
Ravi Prakash, Dipankar Chugh and Karan V. I. S. Kaler
Department of Electrical and Computer Engineering, Schulich School of Engineering, University of Calgary
2500 University Drive NW, Calgary, (T2N 1N4), Canada
Keywords: Surface Microfluidics (SMF), Dielectrophoresis (DEP), Biochemical Assay, DNA, Lab-on-a-chip, Droplets.
Abstract: Quantitative analysis of chemical and biochemical molecules is an important requirement in many
biochemical assays and can be a challenging task in microfluidic systems due to the smaller sample
volumes. In the present study, we report on the detection and quantification of nucleic acid samples
contained in nanoliter (nL) and picoliter (pL) droplets, formed by employing a DEP based surface
microfluidic system. This surface microfluidic system utilizes non-uniform AC electric fields for dispensing
multiple, nanoliter (and picoliter) sized aliquots of samples and reagents, which can furthermore be
individually addressed, transported and mixed on-chip in a controlled and parallel fashion. Quantification of
dsDNA samples is carried out using a fluorescence based Quant-IT
™
PicoGreen
®
assay, performed on the
surface microfluidic chip, while the low-level fluorescence emissions are quantified using a photo-multiplier
tube. Our findings show that sample DNA concentrations remain uniform across the dispensed droplets,
although the volume of droplets can be varied as per requirements. Experimental results furthermore prove
that our DEP based and electric field assisted on-chip mixing methodology is at par with conventional
mixing strategies such as vortexing, stirring etc. and more readily achieved compared to conventional closed
channel microfluidic systems.
1 INTRODUCTION
Advances in microfabrication of microfluidic
devices has given rise to the capability of
performing biochemical assays and analysis using
small amount of samples, giving rise to the concept
of lab-on-a-chip (LOC), in which sample pre-
treatment, transportation, reaction, separation and
detection can be achieved on a common platform.
These microfluidic LOC devices provide significant
advantages over conventional approaches in
handling, processing and sample analysis. The
miniaturization specifically provides low
consumption of reagents and samples, portability,
low power consumption, high throughput screening,
disposability, low cost and potential for automated
and remote operation as a result of further system
level integration.
Until recently, such microfluidic devices have
employed microchannels etched in glass, silicon or
other polymeric materials, to handle and process low
amounts of fluidic samples, aided by valves and
pumps to transport the sample or reagents (Hong and
Quake 2003). In contrast, a new class of surface
microfluidic (SMF) devices have emerged, which
have circumvented the need for microchannels to
confine fluids and furthermore require no external or
on-chip pumping or valving. These SMF devices
employ AC electric fields, typically in the low to
mid-frequency regions of the frequency spectrum, to
manipulate aqueous dielectric media, in form of
droplets, on top of suitably tailored hydrophobic
surfaces. The actuation and manipulation of liquids
and droplets in these devices specifically leverage
the motive forces of dielectrophoresis (DEP) or
Electrowetting (EW) to affect sample handling and
offer an attractive alternative to conventional closed
channel microfluidic devices, by providing high
speed dispensing of multiple, equi-volume,
nanoliter sized sample droplets (Pollack et al. 2002,
Jones 2001).
More recently, Chugh and Kaler have shown the
integration of Liquid DEP (L-DEP) based droplet
dispensing with the Droplet DEP (D-DEP)
conveyance scheme to demonstrate automated
binary mixing of two different equi-volume liquid
sample droplets at specific reaction sites (Chugh and
51
Prakash R., Chugh D. and V.I.S. Kaler K. (2010).
QUANTITATIVE BIOCHEMICAL ASSAY ON A SURFACE MICROFLUIDIC DEVICE.
In Proceedings of the Third International Conference on Biomedical Electronics and Devices, pages 51-58
DOI: 10.5220/0002589500510058
Copyright
c
SciTePress

Kaler 2008, 2009). In this article we report on
further enhancements to the L-DEP sample handling
and manipulation scheme, including the ability of
dispensing different quantities of reagents, by
employing a tapered L-DEP electrode structure. The
tapered L-DEP electrodes, detailed in later sections,
were designed to facilitate automated dispensing of
different sets of daughter droplets from the same
parent but differing in volume. This automated
variable volume sample droplet dispensing scheme
is especially beneficial for chip based biochemical
assays that require different amounts of sample or
reagents to be mixed or titrated.
The utility and capabilities of the variable
volume droplet dispensing is further demonstrated
by performing a DNA-PicoGreen® assay, facilitated
by the judicious integration of three sets of fishbone
shaped electrodes, for transporting the dispensed
sample and reagents droplets to specific reaction
sites. The effectiveness of mixing of the DNA and
PicoGreen (PG) sample droplets was quantified by
detecting the fluorescence signal emitted as a result
of DNA-PG binding using a photomultiplier tube
(PMT). Quantitative gradient of DNA sample was
clearly reflected in fluorescent intensity gradient in
different mixed droplets. Further details of the SMF
devices and measurement systems used to validate
the variable volume dispensing and subsequent
manipulation of the daughter droplets is provided in
the materials and methods sections.
2 BACKGROUND
Dielectrophoresis (DEP) is an electromechanical
phenomenon, manifested as result of the interaction
of the spatially non-uniform electric fields with
polarizable materials (Pohl 1978). Non-uniform
electric fields act on polar molecules, impelling
them to regions of higher field strength. Although
the origins of DEP can be dated back to the late 19
th
century (Pellat 1895), it is more recently that DEP
has been usefully employed in SMF devices as a
means of manipulating and transporting polarizable
liquids on-chip (Ahmed and Jones 2006). The liquid
and droplet DEP actuation methodology, underlying
physical principles and electrode design parameters
have been reported previously (Jones et al. 2001,
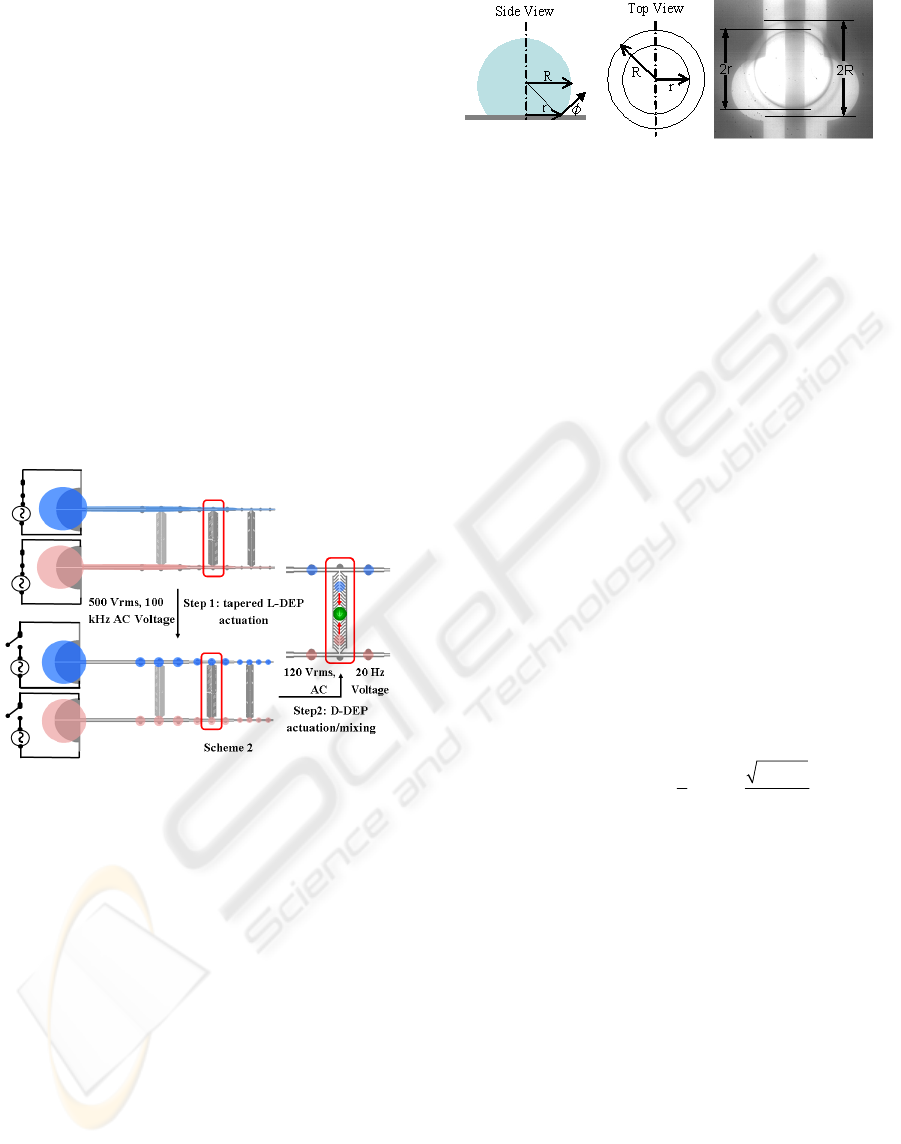
Gunji et al. 2004). In the present study, a tapered L-
DEP electrode structure was utilized for dispensing
variable volume droplets as shown in Figure 2.1
(Chugh and Kaler 2009). These L-DEP electrodes,
serve as a fluidic track for the ‘parent’ sample
droplet pipetted at one end of the structure. On
applying an AC voltage across the coplanar
Figure 2.1: Tapered L-DEP actuation scheme 1.
electrodes, a liquid jet is formed from the sample
droplet and conveyed rapidly towards the opposite
end, covering regions of high field intensity. Once
the jet has reached the opposite end, the applied
voltage is removed and the jet disintegrates into
smaller sized ‘daughter’ droplets at the semi-circular
bump sites, as shown in Figure 2.1. The spacing
between the bumps and jet break-up is governed by
Rayleigh’s instability criteria (
Lord Rayleigh 1879).
Further manipulation and transport of these
dispensed daughter droplets was achieved through
droplet-DEP actuation, developed by (Gunji et al.
2004).
In the present study, we have leveraged liquid
and droplet DEP sample actuation schemes to
dispense, transport, mix nano/picoliter droplets and
execute biochemical assays on our DEP based SMF
device.
3 EXPERIMENTAL
3.1 Device Fabrication
Surface microfluidic devices, used in the present
study, were fabricated using commercially available
4” silicon wafers passivated by 5μm film of SiO
2
.
The device consists of L-DEP and D-DEP
microelectrode structures, fabricated in two different
metal layers (Aluminum) separated by a dielectric
film (600nm thick Si
3
N
4
), sandwiched between them
for electrical isolation. For the ‘bottom’ layer
electrodes, a 200nm thick layer of Al was sputtered
on the passivated silicon substrate. Microelectrode
structures were patterned using standard
photolithography procedure (Thirukumaran and
Kaler 2007). Thereafter, a thin layer of silicon
nitride (600 nm) was deposited on top of the Al
microelectrodes using plasma enhanced chemical
vapor deposition (PECVD) technique. A second
layer of Al (200nm) was sputter deposited and
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
52
patterned to obtain the ‘top’ layer microelectrode
structures, followed by a second Si
3
N
4
deposition
(400 nm) using PECVD. Finally, the top surface of
the microfluidic chip was rendered hydrophobic by
spin coating a thin layer (~ 0.1 μm) of Teflon® AF
2400 (DuPont Inc., USA), which is critical for
reliable D-DEP actuation (Gunji et al. 2004).
3.2 Sample Preparation
Detection and quantification of dsDNA samples was
carried out using a fluorescence based Quant-IT™
PicoGreen
®
assay (Molecular Probes™, Invitrogen,
USA). PicoGreen
®
(PG) is a well-known fluorescent
nucleic acid stain that selectively binds to dsDNA
(Zipper et al. 2004). PG has a large enhancement in
its fluorescence emission on binding to dsDNA, with
excitation and emission maxima at 488nm and
520nm, while unbound PG has virtually no
measurable fluorescence. A 200ng/μL stock DNA
sample was prepared by suspending lyophilized
plasmid DNA samples of pUC57 (GenScript, USA)
in 1mM Tris solution, prepared fresh in de-ionized
(DI) water on the day of the experiment. The pH of
the Tris solution was adjusted to 7.5 using a 50mM
MES solution. Dilutions of the stock DNA sample
were made in Tris-MES solution. Final plasmid
DNA concentrations were measured using a
NanoDrop UV-Visible spectrophotometer (Thermo
Scientific, USA). PG
reagent was supplied as a 1mL
concentrated solution in dimethylsulfoxide (DMSO).
Based on the supplier’s protocol and concentration
of DNA sample used for on-chip assays, a working
sample of the dye was prepared by making a 4 fold
dilution of the concentrated dye (in DMSO) in Tris-
MES solution. A small amount of Tween
®
20 was
added to all samples to minimize surface adsorption.
3.3 Experimental Setup
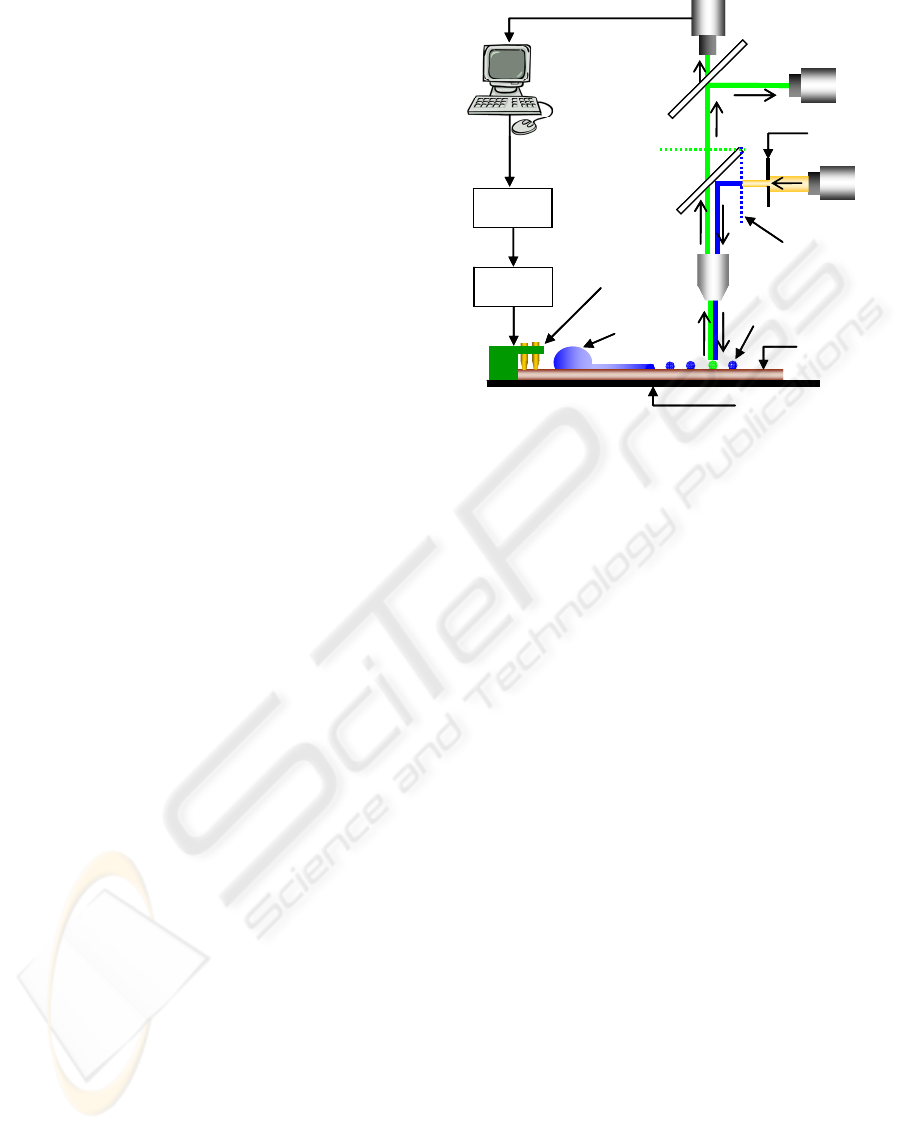
The experimental setup is comprised of a reflected
fluorescence microscope system (BX51, Olympus,
Japan), optically coupled to a CCD color camera
and a Photomultiplier Tube (H7468-01, Hamamatsu,
Japan) to facilitate fluorescence intensity
measurements. A block diagram of the optical setup
is schematically illustrated in Figure 3.3.1. The CCD
camera was replaced by a high-speed camera
(MS70K, Mega Speed, Canadian Photonics Inc.) for
recording Liquid and Droplet-DEP actuations, when
required. Electrical connections to the SMF chip
were enabled using spring loaded contact pins and
high voltage relays (9104 series, COTO Technology,
USA) assembled on a printed circuit board (PCB).
The PCB was mounted on-top of the SMF chip and
CCD
PMT
50/50 beam splitter
ex. filter
470-490 nm
em. filter
λ > 520 nm
dichroic mirror
500 nm
surface
μfluidic chip
parent sample
droplet
20X objective
motorized XYZ stage
Power
Amplifier
Signal
Generator
LabVIEW
Interface
PCB with
contact pins
daughter droplet
Data extraction
field iris
diaphragm
light source
CCD
PMT
50/50 beam splitter
ex. filter
470-490 nm
em. filter
λ > 520 nm
dichroic mirror
500 nm
surface
μfluidic chip
parent sample
droplet
20X objective
motorized XYZ stage
Power
Amplifier
Signal
Generator
LabVIEW
Interface
PCB with
contact pins
daughter droplet
Data extraction
field iris
diaphragm
light source
Figure 3.3.1: Schematic of the experimental setup.
the completed assembly was housed on a motorized
microscope stage (Optiscan
®
ES103, Prior
Scientific, USA).
A signal generator (TGA 1244, TTi, UK) and
high-voltage amplifier (Precision Power Amplifier
5205A, Fluke) provided the AC voltages required
for liquid and droplet actuations. Furthermore, a
software driver, developed in LabVIEW (National
Instruments, USA) was utilized to program the
signal generator for timed and sequential application
of AC voltages and controlling liquid and droplet
DEP actuations.
3.4 Device Operation
In the present study, two different sets of
experiments were performed to investigate (a) the
variations in DNA (pUC57) concentration, in the
individual daughter droplets, dispensed using the
tapered L-DEP electrode structure (scheme 1) and
(b) on-chip droplet dispensing and mixing of DNA
and PG daughter droplets, together with
fluorescence intensity measurements (scheme 2).
For the first set of experiments, utilizing scheme
1, a DNA+PG sample was prepared off-chip, in
micro-centrifuge tube and vortexed to thoroughly
mix the two components. A 1 μL droplet of this
mixed sample was manually pipetted at one end of
the tapered L-DEP electrode as shown in Figure 2.1.
An AC voltage (500 Vrms @ 100 kHz) is briefly
(40–100 ms) applied across the L-DEP electrodes to
dispense nano and picoliter sized ‘daughter’
droplets. The amount of DNA (moles) in each
daughter droplet can be co-related to the intensity of
QUANTITATIVE BIOCHEMICAL ASSAY ON A SURFACE MICROFLUIDIC DEVICE
53
fluorescence emissions observed from the daughter
droplet. Thus to study DNA concentration
uniformity in the dispensed daughter droplets of
different volume, each droplet was individually
observed using the optical set-up illustrated in
Figure 3.3.1. Fluorescent emission from each
daughter droplet is detected and quantified using a
PMT. To ensure consistency and measure low-level
fluorescent emissions from the daughter droplets, the
PMT is operated at a constant high gain value
(
6
0.7 10× ) with a fixed optical path and constant
light intensity. A field iris diaphragm was used to
restrict the diameter of the incident light beam
illuminating the daughter droplets, which excludes
extraneous light from entering the objective, thereby
improving signal to noise ratio (S/N). The iris
aperture was adjusted to circumscribe the largest
daughter droplet (formed on the w = g = 40 μm L-
DEP electrode) and kept same for all other smaller
daughter droplets.
Figure 3.4.1: 1x1 matrix of tapered L-DEP and D-DEP
structures for on-chip assay (Scheme 2).
In the second set of experiments, utilizing
scheme 2, unmixed sample droplets (1 μL) of DNA
and PG are individually pipetted over L-DEP
structure, as shown schematically in Figure 3.4.1.
For executing on-chip sample and reagent mixing,
fishbone shaped D-DEP electrodes are integrated
with the L-DEP electrodes. Daughter droplets of
both DNA and PG are formed by employing the
tapered L-DEP electrode, as mentioned previously.
For mixing the DNA and PG daughter droplets, a
lower frequency AC voltage (120 Vrms @ 20 Hz) is
applied across the D-DEP electrodes, which
transports the daughter droplets from semi-circular
bumps. Selected video frames showing the
integrated liquid and droplet actuation scheme are
illustrated in Figure 4.4.1. All experiments were
performed under a low viscosity silicone oil bath
(200
®
FLUID, 5CST, Dow Corning) to prevent
Figure 3.4.2: Volumetric measurement of daughter
droplets.
rapid evaporation of daughter droplets.
Volumetric measurement for the dispensed
daughter droplets was required in order to correlate
the PMT output (I
P
) with the DNA concentration.
Since the top surface of the SMF chip is coated with
Teflon®, resulting in a highly hydrophobic surface,
droplets dispensed on the surface assume a nearly
spherical shape (contact angle ~110
o
). However,
droplet’s contact angles have strong dependence on
biomolecular species (such as enzymes, proteins,
cells etc.), which can get adsorbed on the surface
and reduce the contact angle (Prakash and Kaler
2008). Thus in order to estimate daughter droplet
volumes, a visual inspection of droplets under high
magnification objective (20X) was performed. A
visual inspection helped distinguish and measure the
two different radii of curvature of the daughter
droplets (r: radius of curvature at the plane where the
droplet contacts the surface and R: actual radius of
the spherical droplet) as shown in Figure 3.4.2. The
measured radii values were used to formulate and
quantify the volume (V) of the daughter droplets
using Eqn. 1.
2
22
23
000
2
sin (1 )
3
R
r
R
r
VrdddrR
R
πϕ
π
θφ
φφθ π
−
===
−
==+
∫∫∫
(1)
4 RESULTS AND DISCUSSION
4.1 Sub-nanoliter Droplet Dispensing
and Bio-sample Quantification
A tapered L-DEP structure (refer scheme1) was used
to dispense droplets of mixed DNA-PG sample with
volumes 2.4 nL, 0.9 nL and 0.25 nL, as shown in
Figure 4.1.1. The droplet volumes were estimated
using equation 1, described above. In order to
confirm that sample concentration remains invariant
for all the dispensed droplets irrespective of the
droplet volume, photocurrent values corresponding
to different sized daughter droplets and 4 different
DNA concentrations were measured and are
summarized in Table 1.
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
54
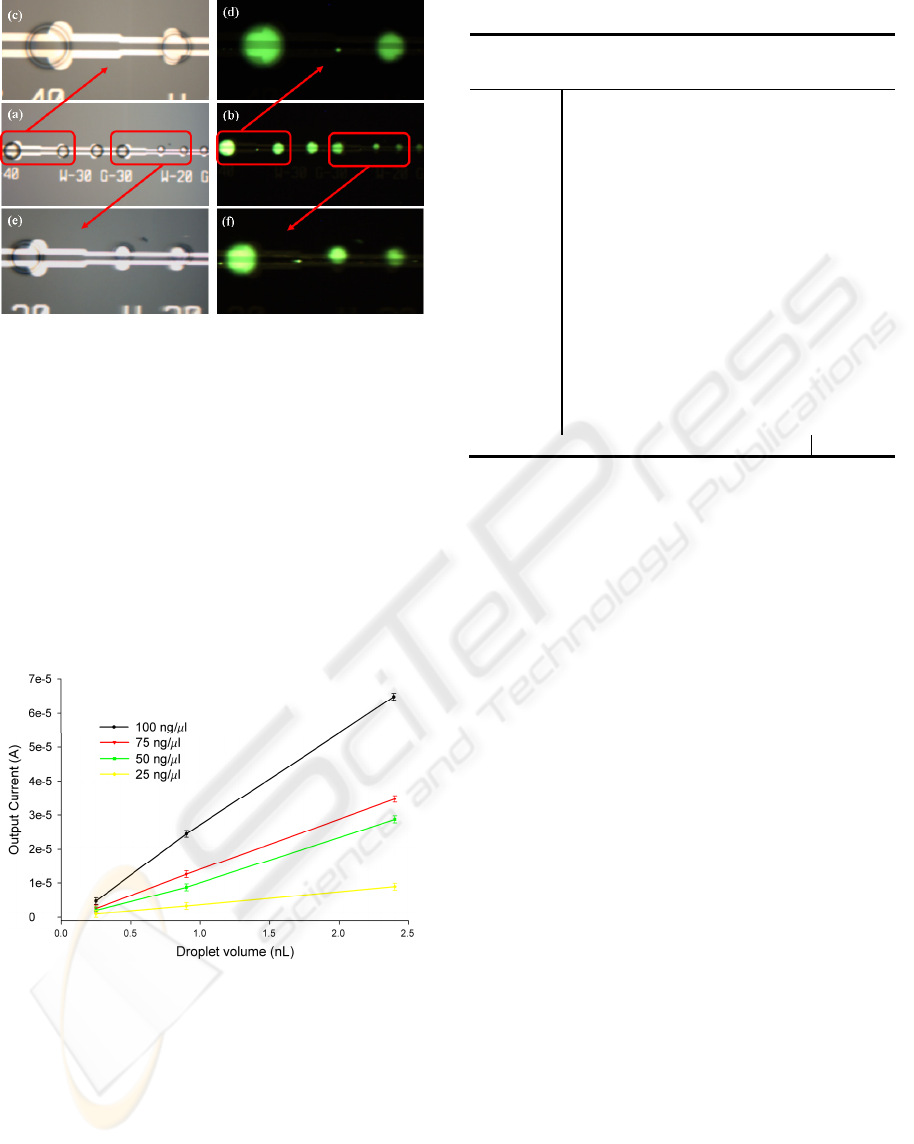
Figure 4.1.1: Bright field and fluorescent images of
different sized daughter droplets containing DNA-PG
complex; (a, b) Bright field and fluorescent image of all
the three sections of tapered L-DEP electrode; (c, d)
Bright field and fluorescent image of 40-30 tapered
electrode section; (e, f) Bright field and fluorescent image
of 30-20 tapered electrode section.
In general, fluorescence emission from the DNA-
PG complex is reported to be linearly proportional to
the quantity of DNA (Singer et al. 1997). Therefore,
since I
P
is directly related to the amount of DNA (or
number of moles) in daughter droplets, the ratio
I
P
/vol. provides a direct measure of sample DNA
molar concentration for each daughter droplet.
Figure 4.1.2: Plots showing PMT output for different sized
daughter droplets of different conc. DNA-PG solutions.
These values, along with the ratio of PMT
photocurrent (I
P
) and droplet volume are tabulated in
Table 1. We found that the ratio (I
P
/vol.) remains
constant for all the different sized daughter droplets
(corresponding to a specific DNA concentration)
confirming that the DNA concentration in each of
these daughter droplets remains invariant. This was
further evident from the plot of output current (I
P
)
vs. measured droplet volume, shown in Figure 4.1.2,
Table 1: Experimental results extracted using scheme 1.
Sample
w = g
(micron)
I
P
(μA)
Vo
(nL)
I
P
/vol.
Correlation
factor
25 ng/μL
DNA with
50X PG
40-40 10.97 2.4 4.57 5.47
30-30 3.99 0.9 4.43 5.65
20-20 1.19 0.25 4.77 5.24
50 ng/μL
DNA with
50X PG
40-40 22.77 2.5 9.11 5.49
30-30 8.76 0.9 9.73 5.14
20-20 2.21 0.25 8.83 5.66
75 ng/μL
DNA with
50X PG
40-40 33.71 2.4 14.05 5.34
30-30 12.57 0.9 13.96 5.37
20-20 3.24 0.25 12.98 5.78
100 ng/μL
DNA with
50X PG
40-40 43.68 2.4 18.20 5.49
30-30 16.53 0.9 18.36 5.45
20-20 4.63 0.25 18.52 5.40
Variance 0.04
where a constant slope (I
P
/vol.) was observed for
each of the four different DNA sample
concentrations (25, 50, 75 and 100 ng/μL).
From this plot and Table 1, we then estimate a
correlation factor to normalize I
P
/vol. ratio against
different DNA concentrations, the inverse of which
provides a measure of photo-current per picogram of
DNA sample. The above findings suggest that using
tapered L-DEP scheme, we can dispense multiple
sample droplets with different amounts (moles) of
DNA sample, while keeping the concentration same
as the parent sample. DNA concentrations were
quantified in the dispensed daughter droplets ranged
from 2.4 nL to 0.25 nL, a capability that is not
readily achieved by today’s microfluidic devices.
4.2 Reliability of the L-DEP Scheme
for Multiplexed Applications
Having shown that DNA concentrations are
invariant for daughter droplets dispensed using L-
DEP actuation, we further assess the reliability and
repeatability of DEP based droplet dispensing
scheme. For this, we conducted several experiments
on simple L-DEP dispensing scheme (scheme 1)
with the same parent sample conc. (50 ng/μL DNA-
PG) to see the reliability and repeatability of our
scheme in dispensing uniform conc. daughter
droplets.
The results of 6 different set of experiments with
the same parent DNA sample droplet over different
but, identical L-DEP electrode structures are shown
plotted in Figure 4.2.1. The results clearly show a
QUANTITATIVE BIOCHEMICAL ASSAY ON A SURFACE MICROFLUIDIC DEVICE
55
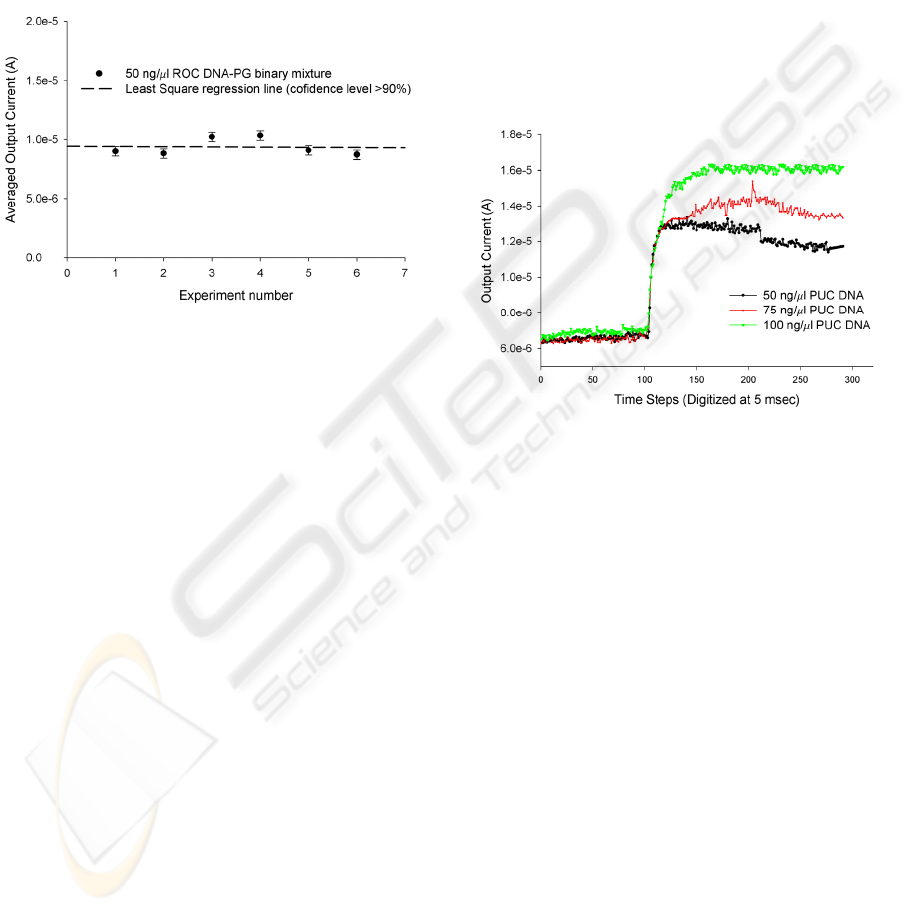
reliable dispensing of 6-8 daughter droplets with
equal concentration (confidence of fit ~92.5%).
The high speed of L-DEP actuation (40 msec for
dispensing an array of sub-nanoliter droplets) and
the hydrophobic Teflon® coated surface minimizes
surface adsorption (Prakash and Kaler 2008) for
biological sample and the accuracy in the size and
concentration of dispensed daughter droplets makes
the scheme suitable for use in an on-chip
multiplexed biochemical assay device.
Figure 4.2.1: Plot showing repeatability of L-DEP based
sub-nanoliter droplet dispensing scheme.
4.3 On-chip Mixing of Constant
Volume Sample and Reagent
Droplets using D-DEP
One of the objectives of our DEP based SMF device
is to extend the idea of mixing to implement chip-
based multiplexed assays where several different
samples and reagents are mixed in all possible
combinations in a parallel, automated fashion.
Effective mixing of samples and reagents is an
essential step involved in all chemical and
biochemical applications. Mixing on-chip is not as
readily achieved compared to macroscopic mixing
of samples, where vortexing or stirring actions could
be easily exploited. This is due to the small size, low
sample/reagent volumes and laminar flow
characteristics of the conventional closed-channel
microfluidic devices resulting in a slow and
diffusion limited mixing. In contrast, SMF devices
are capable of mixing sub-nanoliter volumes of
sample and reagent droplets more efficiently (Fair et
al. 2003). We use D-DEP actuation, where electric
field mediated stirring action (electroconvective
effects) takes place during droplet transportation and
facilitates mixing once the droplets come in contact
and merge. To demonstrate this, we used an
integrated Liquid and Droplet-DEP structure (shown
boxed in Figure 3.4.1) which was elaborated in the
experimental section. Three different concentrations
of the DNA sample along with PG dye were used.
Roughly 0.6 nL volume of DNA sample and the PG
dye was first dispensed using the L-DEP dispensing
scheme. The DNA sample and PG daughter droplets
thus formed were then moved towards the
reaction/mixing site using D-DEP actuation.
Fluorescence emissions were recorded in real-time
as the two daughter droplets mixed and are shown in
Figure 4.3.1. We observed a steady increase in the
PMT output which indicates that all the three
different conc. of DNA sample demonstrate a nearly
similar mixing or, ligand binding kinetics (evident
from the identical slope of PMT current vs. time
curves).
Figure 4.3.1: Plot showing time domain data extracted
from PMT illustrating binary mixing of sample and
reagent daughter droplets.
The PMT output finally saturates and remains
constant, indicating complete and thorough mixing
of DNA sample with the PG reagent. The entire
mixing assay was complete within 4-5 sec from
manual pipetting of the parent sample droplets.
These results clearly suggest that on-chip mixing
can be more readily achieved using our DEP based
SMF device as compared to conventional closed-
channel microfluidic devices which require large
sample volumes (in mL), longer (in mm) and wider
mixing channels, larger mixing time and furthermore
sophisticated pump and valve arrangements (Park et
al. 2006).
4.4 On-chip Variable Volume,
Multiplexed DNA-PicoGreen®
Assay
Having demonstrated the capability of our SMF
device in achieving some of the key sample handling
requirements for an on-chip assay system including,
(1) dispensing arrays of sub-nanoliter sample and
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
56
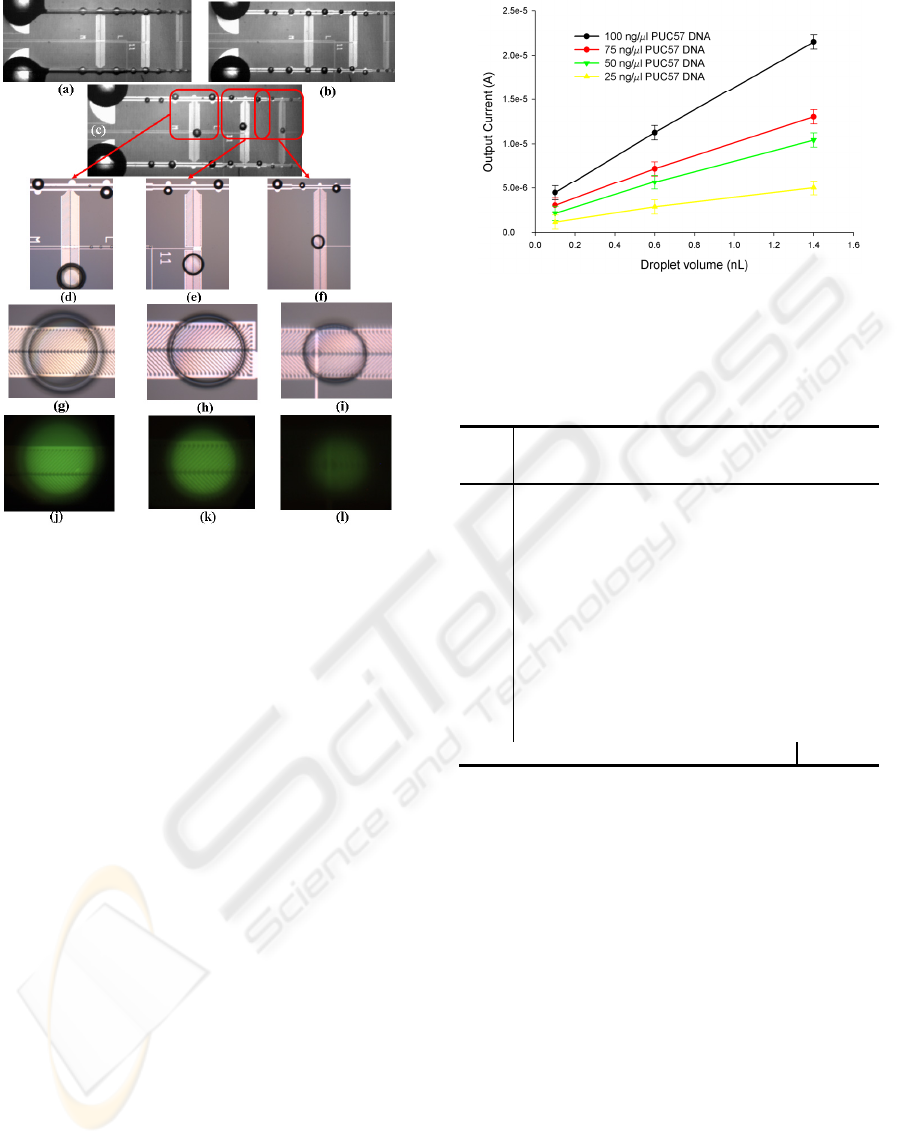
Figure 4.4.1: Micrographs demonstrating results of on-
chip DNA quantification assay; (a, b)L-DEP actuation and
droplet dispensing; (c) mixed daughter droplets; (d, e, f)
Unmixed DNA sample droplets (along tapered L-DEP
electrodes) and mixed DNA-PG droplets; (g-l) bright field
and fluorescent images of the mixed DNA-PG droplets
showing the fluorescent intensity gradient.
reagent droplets with controllable sample mass and
uniform concentration, and (2) efficient mixing of a
pair of dispensed sample and reagent droplets, we
now demonstrate an on-chip nucleic acid assay by
implementing a 1x1 matrix of tapered L-DEP and D-
DEP electrode structures illustrated in scheme 2
(Figure 3.4.1).
Four different concentrations (25, 50, 75 and 100
ng/L) of DNA sample were actuated over one of
the two tapered L-DEP electrodes to dispense
multiple daughter droplets with uniform
concentrations but varying volumes and different
moles of DNA. On the other tapered L-DEP
electrode, PG sample was actuated in a similar
fashion.
Three different sets of paired fishbone electrodes
were used to simultaneously transport sample and
reagent droplets from each of the three different
steps of the tapered L-DEP electrode structure to the
corresponding reaction sites under the influence of
an externally applied electric field, to facilitate
DNA-PG sample mixing (Figure 3.4.1). The entire
process was conducted within 2-4 seconds of
actuation (Figure 3.4.1 and Figure 4.4.1).
Figure 4.4.2: Plots showing PMT output for on-chip DNA-
PG assay using experimental scheme 2, for different DNA
sample concentrations.
Table 2: Results extracted from on-chip DNA-PG assay
experiments employing scheme 2.
DNA
conc.
PG
w = g
(micron)
I
P
(μA)
vol.
(nL)
I
P
/vol.
Correlation
factor
25
ng/μL
50X
40-40 5.96 1.4 4.26 5.87
30-30 2.78 0.6 4.63 5.40
20-20 1.13
0.1
4.51 5.54
50
ng/μL
40-40 12.44 1.4 8.88 5.63
30-30 5.49 0.6 9.15 5.46
20-20 2.15
0.1
8.60 5.81
75
ng/μL
40-40 17.85 1.4
12.7
5.88
30-30 7.71 0.6
12.8
5.83
20-20 3.25
0.1 13.0
5.77
100
ng/μL
40-40 24.50 1.4
17.5
5.71
30-30 11.26 0.6
18.7
5.33
20-20 4.47
0.1 17.8
5.59
Variance 0.04
The PMT output current (I
P
) corresponding to the
mixed droplets was plotted against the volume of the
individual DNA daughter droplets, measured prior to
mixing and shown in Figure 4.4.2. The ratio I
P
/vol.
correlates to the DNA concentration in the dispensed
daughter droplets. Constant slopes for each of the
different DNA concentrations used, indicate that the
ratio of output current (I
P
) and DNA droplet volume
and hence DNA concentration, remains constant for
each of the mixed droplets. Ip/vol. values and the
corresponding correlation factor for on-chip DNA-
PG assay are reported in Table 2. These values of
correlation factor reported in Table 2 are
furthermore in good agreement with the values of
correlation factor reported in Table 1 for the
corresponding off-chip mixed DNA-PG droplets,
suggesting that on-chip quantification assay is
successfully achieved.
QUANTITATIVE BIOCHEMICAL ASSAY ON A SURFACE MICROFLUIDIC DEVICE
57

5 CONCLUSIONS
In this study, we have successfully demonstrated the
utility of a tapered electrode structure to
dielectrophoretically dispense variable volume
nanoliter to sub-nanoliter sample droplets (2.4 nL to
0.25 nL) on top of hydrophobic surfaces, with
precision. This tapered droplet scheme was
furthermore interfaced with a fishbone droplet
conveyance scheme to demonstrate its utility in
performing a quantitative, multiplexed assay.
The fluidic sample handling capabilities of the SMF
devices reported in this article may be potentially
leveraged for several purposes including drug
discovery, genomics and pathogen detection. This
SMF scheme can also be multiplexed to an m
×
n
matrix to achieve HTS capabilities as an alternative
to the existing close channel technology (Thorsen et
al. 2002). The oil bath submerged experimental
setup can be replaced by using the sub-nanoliter
emulsion dispensing scheme, reported by Prakash
and Kaler (2009).
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial
support provided by National Science and
Engineering Research Council of Canada (NSERC),
CMC Microsystems and Micralyne Inc. (Canada) in
support of the research work detailed in this article.
Authors furthermore acknowledge the assistance
provided by the Nanofab staff at U of Alberta in
fabricating the SMF devices.
REFERENCES
Ahmed R. and Jones T.B., 2006. Dispensing picoliter
droplets on substrates using dielectrophoresis. J.
Electrostat 64:543–549.
Chugh D. and Kaler K.V.I.S.,2009. Microfluid Nanofluid,
DOI 10.1007/s10404-009-0469-7.
Chugh D. and Kaler K.V.I.S., 2008. Integrated liquid and
droplet DEP for lab-on-a-chip applications.
Proceedings of 12th international conference on
miniaturized systems for chemistry and life sciences,
San Diego, pp 733–735.
Gunji M., Nakanishi H. and Washizu M., 2004. Droplet
actuation based on single-phase electrostatic
excitation. Proceedings of Micro Total Analysis
Systems, Malmo, pp 168–170.
Hong J.W. and Quake S.R., 2003. Integrated nanoliter
systems. Nat. Biotechnology, 21:1179–1183.
Jones T.B., 2001. Liquid dielectrophoresis on the
microscale. J. Electrostatics, 51–52:290–299.
Jones T.B., Gunji M., Washizu M., Feldman M.J., 2001.
Dielectrophoretic liquid actuation and nanoliter
droplet formation. J. Appl. Phys, 89(3):1–8.
Lord Rayleigh, 1879. On capillary phenomena of jets.
Proc. Roy. Soc. (London), 27: 71–97.
Paik P., Pamula V.K., Pollack M.G. and Fair R.B., 2003.
Electrowetting-based droplet mixers for microfluidic
systems. Lab Chip, 3, 28–33.
Park H.Y., Qiu X., Rhoades E., Korlach J., Kwok L.W.,
Zipfel W.R., Webb W.W. and Pollack L., 2006.
Achieving Uniform Mixing in a Microfluidic Device:
Hydrodynamic Focusing Prior to Mixing. J. Anal.
Chem., 78, 4465-4473.
Pellat H., 1895. Mesure de la force agissant sur les
dielectriques liquids non electrises places dans un
champ elitrique. C R Acad Sci, Paris 119:691–694.
Pohl H.A., 1978. Dielectrophoresis, Cambridge University
Press, Cambridge.
Pollack M.G., Shenderov A.D. and Fair R.B., 2002.
Electrowetting-based actuation of droplets for
integrated microfluidics. Lab Chip, 7152:96–101.
Prakash A. R., Amrein M. and Kaler K. V. I. S., 2008.
Characteristics and impact of Taq enzyme adsorption
on surfaces in microfluidic devices. Microfluid
Nanofluid, 4, 295–305.
Prakash R. and Kaler K.V.I.S., 2009. DEP actuation of
emulsion jets and dispensing of sub-nanoliter
emulsion droplets. Lab Chip, Vol. 9:2836–2844.
Singer V.L., Laurie J.J., Yue S.T. and Haugland R.P.,
1997. Characterization of PicoGreen reagent and
development of a fluorescence-based solution assay
for double-stranded DNA quantitation. Anal Biochem,
249:228–238.
Thirukumaran T.K. and Kaler K.V.I.S., 2007. Surface
microfluidics—high-speed DEP liquid actuation on
planar substrates and critical factors in reliable
actuation. J. Micromech Microeng, 17:743–752.
Thorsen T., Maerk S.J. and Quake S.R., 2002.
Microfluidic large-scale integration. Science, 298:580–
584.
Zipper H., Brunner H., Bernhagen J. and Vitzthum F.,
2004. Investigations on DNA intercalation and surface
binding by SYBR Green I, its structure determination
and methodological implications. Nucleic Acids
Research, Vol. 32, No. 12.
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
58
