
Communicating Personalized Risk Factors for Lifestyle Coaching
George Drosatos
1
, Kyriakos Bakirlis
2
, Pavlos S. Efraimidis
2
and Eleni Kaldoudi
1
1
School of Medicine, Democritus University of Thrace, Dragana, Alexandroupoli 68100, Greece
2
Department of Electrical and Computer Engineering, Democritus University of Thrace, Kimmeria, Xanthi 67100, Greece
Keywords: eHealth, Personal Health Systems, Risk Factors, Healthy Behaviour, Lifestyle Related Disease, Mobile
Application, Privacy by Design.
Abstract: Chronic non-communicable diseases such as diabetes, chronic cardiorenal and respiratory disease and
cancer, are serious, burdensome and costly conditions that share a common characteristic: they heavily
depend on common behavioural risk factors, such as physical activity, diet, stress, and substance abuse.
Despite concerted efforts it has been remarkably difficult to change such lifestyle related disease
determinants, as behavioural change is a complex process requiring significant personal responsibility. In
this paper we propose a personal mobile eHealth application to communicate personalized lifestyle related
health risks and understand their individual impact on personal health condition and disease progression.
1 INTRODUCTION
Lifestyle related diseases are multifactorial, chronic,
costly diseases that create a world-wide burden on
individuals, and society. However, they share a
common characteristic: underlying disease
determinants hugely depend on lifestyle choices and
environmental factors, thus can be modified so that
disease can be prevented and its progression can be
halted.
Current trends in health informatics reflect an
important contemporary shift towards citizen
engagement for health, wellbeing and disease
prevention, as opposed to disease management.
However, broadcasting generic health messages (e.g.
‘do this, don’t do that’) has limited effects unless
there is a convincing, easily perceived and
personally customized body of evidence to back
healthy choices.
In this paper we propose a novel mobile eHealth
application to communicate personalized health risks
and coach the user to understand their impact on
various aspects of personal health and plan changes
in lifestyle related risk factors. The proposed
personal application consumes publicly available
open data on health risk evidence via standardized
programming interfaces and meshes these data with
private personal health information. The output is
graphical interactive representation of common
health risks as personalized to each individual.
Privacy is engineered into the design of the
application. The ultimate goal is to provide the
means to foster understanding of the complex
interdependent nature of personal lifestyle choices as
a disease determinant.
2 BACKGROUND
In the second global status report in a triennial series
published in 2014, WHO reports that chronic non-
communicable diseases such as cardiovascular
disease, cancer, diabetes and chronic respiratory
disease, were responsible for 38 million deaths per
year (68% of all deaths) and are projected to
increase from 38 million in 2012 to 52 million by
2030 (WHO, 2014).
For example, the total number of people with
diabetes has risen from 108 million in 1980 to 442 in
2014 (WHO, 2014), reaching epidemic proportions
globally. Diabetes is profoundly impacted by
lifestyle options including dietary intake, exercise,
stress, sleep, and use of alcohol. Interventions which
change each of these lifestyle behaviours are shown
to improve the health of diabetic patients (Spruijt-
Metz, 2014).
Another major category of life style related
diseases includes cardiovascular diseases, including
heart attacks, strokes, coronary heart disease,
cerebrovascular disease and peripheral arterial
Drosatos, G., Bakirlis, K., Efraimidis, P. and Kaldoudi, E.
Communicating Personalized Risk Factors for Lifestyle Coaching.
DOI: 10.5220/0006660405710578
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 5: HEALTHINF, pages 571-578
ISBN: 978-989-758-281-3
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
571

disease. Behavioural risk factors are responsible for
about 80% of cardiovascular disease instances4 and
include unhealthy diet, physical inactivity, and
tobacco and alcohol use. Overall cardiovascular
disease is currently estimated to cost the EU
economy almost €210 billion a year; of the total cost
of cardiovascular disease in the EU, 53% (€111
billion) is due to health care costs, 26% (€54 billion)
to productivity losses and 21% (€45 billion) to the
informal care of people with cardiovascular disease
(European Heart Network, 2017).
Therefore, WHO has developed programmes to
reduce lifestyle related disease incidence by limiting
risk factors such as tobacco, physical inactivity,
dietary factors, obesity and overweight, alcohol use,
and environmental pollution. Overall, large, multicen-
tre cohort studies (Knoops, 2004; Moreno, 2008;
Haveman-Nies, 2001) have shown that even small
changes in lifestyle can make an important difference
towards health improvement and disease reduction.
Changing such lifestyle related disease
determinants represents the single biggest
opportunity to improve health outcomes while
bringing costs under control. But the ‘easy stuff’ is
far from easy. Despite concerted efforts by policy
makers, providers and payers — not to mention the
best intentions of individuals — it has been
remarkably difficult to effect behavioural change
(Ernst & Young, 2012).
Behavioural change is a complex process
requiring significant personal responsibility. Without
personalized predictive information, it is not
possible to leverage on this. It is also important to
understand your health risks in order to benefit from
news and research about specific diseases and plan
preventing monitoring.
2.1 Risk Evidence in Medicine
A health risk factor is a variable (demographic,
genetic, behavioural, medical, or even environmental)
which when present in an individual increases the
probability of a (usually) negative outcome to occur.
This risk association between the exposure agent and
the outcome is conveyed via a relative risk value.
For example, medical evidence suggests that
obesity is a risk factor for coronary arterial disease
(Guh, 2009). In this particular systematic review and
meta-analysis, men with a body-mass index (BMI)
between 25 and 30 kg/m
2
were found to have a
relative risk ratio of 1.29 to develop coronary arterial
disease (as compared to normal male of a BMI 18.5
to 25 kg/m
2
). A different risk association (for the
same risk factor) is found for men of a BMI greater
than 30 kg/m
2
, who present an elevated relative risk
ratio of 1.72. The same evidence source shows that
women with a BMI between 25 and 30 kg/m
2
have a
risk ratio of 1.80 to develop coronary arterial disease
and when their BMI is above 30 kg/m
2
the risk ratio
is elevated to 3.10. Thus, in this example, four
different associations are described between obesity
(and age) and the outcome of coronary arterial
disease.
Risk factors are derived from population
statistical studies. Systematic reviews select similar
studies and via statistical meta-analysis combine
results to improve the estimates of risk ratios and
increase the level of evidence. Several risk
associations are then used to build total risk
estimation models for the prediction of certain
outcomes that are affected by multiple risk factors at
the same time. For example, in cardiovascular
disease, several estimation systems exist, that vary
considerably in terms of the population size and
characteristics, their statistical considerations,
validation, and set of risk factors considered, e.g.,
cardiovascular risk, include the Framingham
equation (Sheridan, 2003), the Joint British Societies
(JBS) formula (Boon 2014), the ASSIGN score
(Woodward, 2007) and the SCORE risk charts
(Perk, 2012).
Conventionally, risk factors form the basis of
medical guidelines and are routinely communicated
to health care professionals to formulate the basis of
clinical patient management.
Evidence on risk is customarily published in
medical scientific press. Indicative of current
research emphasis on health risk prediction is the
exponential increase of published research papers in
the field. To illustrate this, we have placed a crude
PubMed query on risk prediction as follows:
"health risk appraisal"[TIAB] OR
"prediction rule"[TIAB] OR
"prediction rules"[TIAB] OR
"prediction model"[TIAB] OR
"prediction models"[TIAB] OR
"prediction score"[TIAB] OR
"prediction scores"[TIAB] OR "risk
score"[TIAB] OR "risk scores"[TIAB]
OR "risk factor"[TIAB] OR "risk
factors"[TIAB]) AND
("0001/01/01"[PDAT]:
"2016/12/31"[PDAT])
To derive the total number of PubMed
publications, we placed the following query:
"0001/01/01"[PDAT]:
"2016/12/31"[PDAT]
HEALTHINF 2018 - 11th International Conference on Health Informatics
572
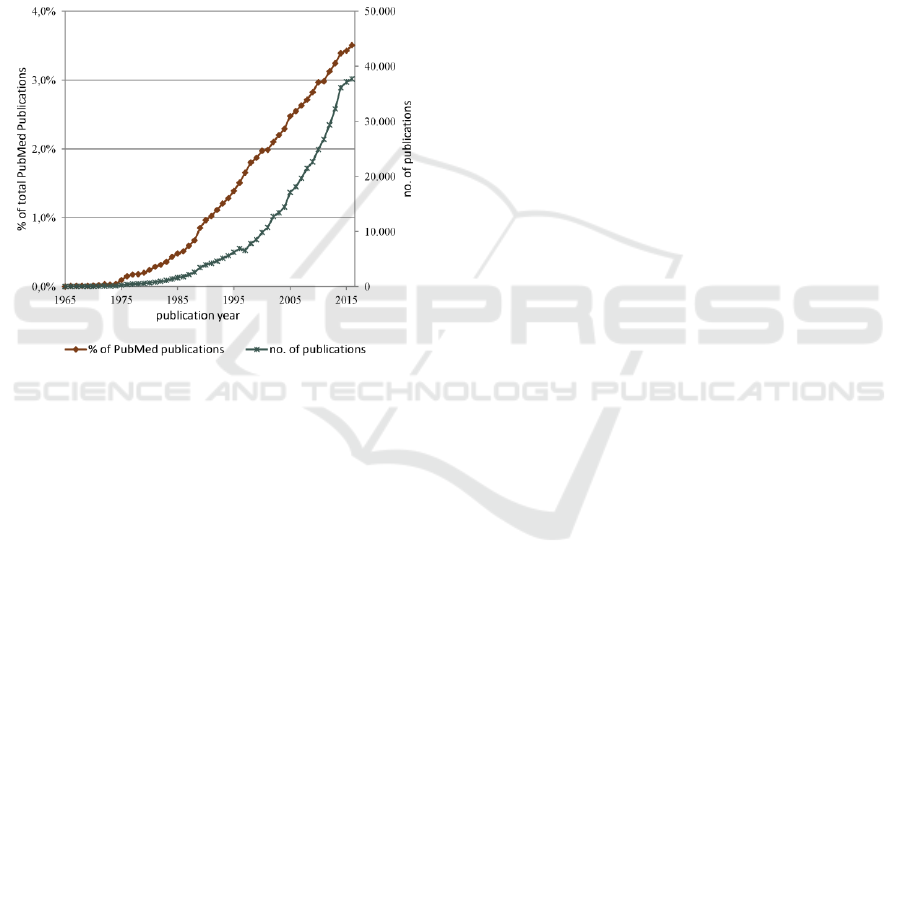
Figure 1 shows the number of retrieved
publications per year plotted as a percentage of the
total number of PubMed indexed publications and as
an absolute number. The query retrieved more than
460.000 related publications that amount to almost
2% of the total PubMed indexed publications.
Overall, there is an increase in the absolute number
of retrieved publications over the years. When seen
as the percentage of the overall PubMed corpus
growth, linear regression for the last 40 years shows
a statistically significant increasing trend (regression
coefficient = 0.000898, p-value < 0.001, R-squared
= 98.5%).
Figure 1: Number of PubMed indexed publications per
year retrieved via the query: “health risk appraisal” OR
“prediction rule” OR “prediction rules” OR “prediction
model” OR “prediction models” OR “prediction score”
OR “prediction scores” OR “risk score” OR “risk scores”
OR “risk factor” OR “risk factors” in the fields of title and
abstract, plotted as a percentage of the total PubMed
indexed publications and as an absolute number.
A number of models have been proposed for
capturing various aspects of the clinical research
process that aims to generate new risk evidence.
Examples include the Ontology-Based eXtensible
data model (OBX) (Kong, 2011) and the Ontology
of Clinical Research (OCRe) (Sim, 2014); both aim
to support the process of generating new scientific
knowledge in medicine, rather than actual evidence
on risk prediction. Scientific knowledge on risk
factors is captured by CARRE risk factor ontology
(Third, 2015). This ontology has been used to create
an open data repository of structured information on
risk factor evidence in the medical domain of
cardiorenal disease and comorbidities, as part of a
European Commission funded project CARRE
(FP7-ICT-611140). The repository was developed
by medical experts following a methodology based
on selecting recent highest evidence sources (meta-
analyses where available) and allowing for peer
review of manually entered risk factor data. This
RDF repository is freely available via a SPARQL
endpoint, and currently holds structured descriptions
of more than 100 risk factors, corresponding to a
total of 250 different risk associations between more
than 50 medical conditions.
2.2 Risk Prediction and Patient
Empowerment
Recent years have seen a paradigm shift towards
patient centred care and health management. Under
this view, patient empowerment has emerged as an
approach that can help improve medical outcomes
while lowering costs of treatment by facilitating self-
directed behaviour change. The concept seems
particularly promising in the prevention and
management of chronic diseases, and it is directly
connected with personalized patient services,
education and preventive measures.
At the First European Conference on Patient
Empowerment held in Copenhagen, Denmark on
11–12 April 2012 (WHO, 2012), R. Johnstone of the
International Alliance of Patient Organizations
claimed that: “What needs to happen is for doctors
to come down off their pedestal and for patients to
get up off their knees”. Currently, the research
community appreciates that improving a person's
ability to understand and manage his or her own
health and disease, negotiate with different teams of
health professionals, and navigate the complexities
of health systems is crucial to achieving better health
outcomes (The Lancet Editor, 2012).
A recent pilot study deployed a self-monitoring
intervention for cardiorenal patients coupled with
rich health risk visualizations as derived from the
CARRE repository (Zhao, 2017). The intervention
requires the patient to use a set of personal and
wearable sensors to monitor health status and
couples these real-time data with medical
information retrieved from personal health records.
Personal private data are then coupled with risk
evidence to create medical alerts for the
management and prevention of disease progression
in cardiorenal patients or those at risk of this disease.
A randomized controlled trial (Kizlaitis, 2016)
showed that the intervention statistically increased
empowerment by a 12.4% in metabolic syndrome
patients, and health literacy by 21.3% in patients
with heart failure of chronic kidney disease.
Communicating Personalized Risk Factors for Lifestyle Coaching
573

3 COMMUNICATING
PERSONALIZED HEALTH
RISKS
In this paper we propose a personal eHealth
smartphone application to communicate health risks
to healthy citizens or people at increased risk of
chronic disease. The aim is to provide a personalized
assessment of lifestyle related risks that create the
necessary knowledge background for people to
make educated choices towards healthier lifestyle
adjustments. To achieve this goal, the Risk Coach
application merges open data on health risk evidence
with private health related information.
The core of the application is the current
evidence on health risks. Such evidence is extracted
from the public CARRE risk factor repository
available at https://devices.duth.carre-project.eu/
sparql. Data on risk evidence is in a structured RDF
format and contains information on exposure and
outcome, risk ratio value (with confidence intervals)
and the conditions under which this risk ratio value
is valid. This condition is based on the
characteristics of the population group used to study
the particular risk factor and also includes levels of
exposure. For example, the repository contains
evidence on obesity as a risk factor for cholelithiasis
as reported in a recent meta-analysis (Guh, 2009).
Evidence on this risk factor reveals four different
risk associations, each corresponding to a different
condition and presenting different risk ratio values
(Table 1). In this example, the condition under
which obesity is a risk factor for cholelithiasis is a
combination of the sex and the value of body mass
index (BMI). It is evident that obese females (body
mass index ≥ 30) are at an increased risk of
cholelithiasis (relative risk of 2.32) as compared to
overweight females (25 body mass index < 30)
and as compared to obese males, who appear to have
about the same risk as overweight females. The
exact form of the condition (the variables and their
values) depends on the clinical study and its
population groups. In fact, risk associations in
CARRE repository at present use a total of 95
different variables to formulate conditions, and this
number will most likely increase as new risk factors
are entered in the repository.
In order to personalize risk communication, the
appropriate personal information needs to be known
and compared to the known risk factor conditions in
the public repository. This is ensured via a Risk
Coach application module that allows the user to
input appropriate personal information. To increase
efficacy and reduce privacy concerns, the
application dynamically recognizes different
variables available in the CARRE risk factor
repository and actively builds the personal
information input fields at real time.
By default, the user is required to input some
basic demographic and biometric information such
as age, sex, height and weight. This initial
information is used to calculate any personal risks, if
these apply based on the specific values describing
the user. To investigate whether further risk factors
apply for the particular user, the application queries
the external risk factor repository for all known
variables and dynamically constructs the appropriate
fields for the user to fill in personal values. Personal
risks are calculated by checking which of the risk
association conditions available in the external
database are valid for the user inserted personal data.
Table 1: A risk factor description example showing four different risk associations for the same risk factor, each
corresponding to a different condition and presenting different risk values (main description fields included).
Description fields
Risk association #1
Risk association #2
Risk association #3
Risk association #4
Risk factor:
obesity
[is an issue in]
cholelithiasis
obesity
[is an issue in]
cholelithiasis
obesity
[is an issue in]
cholelithiasis
obesity
[is an issue in]
cholelithiasis
Condition:
(body mass index ≥ 25
AND body mass index
< 30)
AND sex = 'male'
body mass index ≥ 30
AND sex = 'male'
(body mass index ≥
25 AND body mass
index < 30)
AND sex = 'female'
body mass index ≥ 30
AND sex = 'female'
Ratio type:
relative risk
relative risk
relative risk
relative risk
Ratio value:
1.09
1.43
1.44
2.32
Confidence Interval:
0.87 – 1.37
1.04 – 1.96
1.05 – 1.98
1.17 – 4.57
PubMed ID:
19320986
19320986
19320986
19320986
HEALTHINF 2018 - 11th International Conference on Health Informatics
574
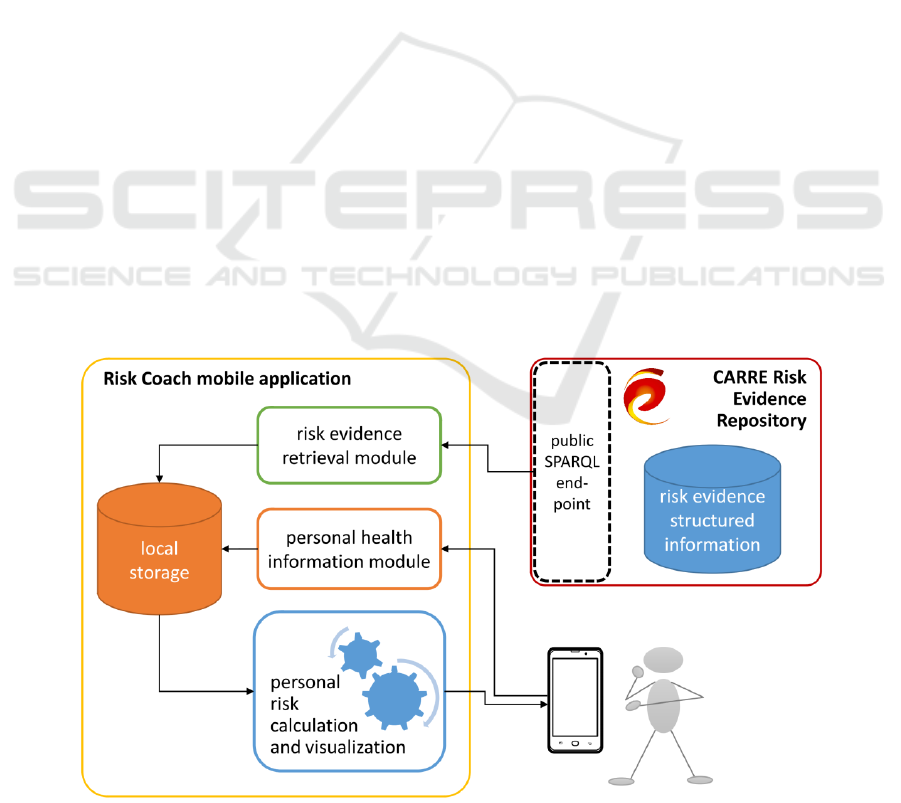
3.1 An Architecture to Preserve
Privacy by Design
The Risk Coach application is designed to be used
by the citizens for self-management of health and
disease mainly outside the formal healthcare context.
Based on the requirements for personal data
communication and an analysis of privacy issues in
personal e-health systems (Drosatos, 2016), we can
identify the following functionalities that may
introduce privacy issues in Risk Coach application:
(1) personal data storage and processing; (2)
integration of personalized public data.
When both storage and processing of personal
data are located on a user personal device, privacy
can generally be achieved by default. This approach
is followed in Risk Coach where personal data and
personal risk calculations are stored locally on the
application. Also, all risk calculation is performed
locally on the smartphone device, as shown in
Figure 2 which presents an overview of the
application architecture. factors.
A potentially important and usually elusive
privacy issues may arise when at run time public
data are requested from external sources (in this case
the risk factor repository). Although the data are
publicly available, just the act of linking particular
data to a specific user may cause a privacy violation,
by revealing the user’s health condition. There are a
number of proposed techniques to conceal user
requirements by altering the initial request, e.g. by
expanding and generalizing the request for public
data. These techniques fetch a large amount of data
to the user application and then a second round of
local processing extracts the specific data relevant to
the user (Efraimidis, 2016). Other emerging
approaches require the cooperation of a group of
users in the system to conceal one another’s requests
(Romero-Tris, 2015). An alternative is to use
anonymous network technologies that protect the
physical address of user from the public service. A
representative example is the TOR service
(Dingledine, 2004), which creates a network of
proxies over the internet and allows recursive
message encryption along the chain of proxies. In
the current approach, the entire risk factor dataset is
locally retrieved, so as to hide user specific requests.
3.2 Implementation
The Risk Coach application is currently implemented
for Android mobile devices using Android Studio
(version 2.3.3) and it is compiled for Android 7.1
(API level 25) with backward compatibility until the
Android 4.4w (API Level 20). Animated graphs are
produced using the MPAndroidChart library
(https://github.com/ PhilJay/MPAndroidChart) and
the retrieval of data throw the CARRE SPAQL
Endpoint is performed using the OkHttp library
(https://square.github.io/ okhttp/).
The application supports the following main
functionalities (implemented as individual Android
application fragments): (a) visualize the generic risk
factor evidence available in the public CARRE risk
factor repository, as an overall repository summary
Figure 2: An overview of the Risk Coach mobile application functional modules and architecture.
Communicating Personalized Risk Factors for Lifestyle Coaching
575
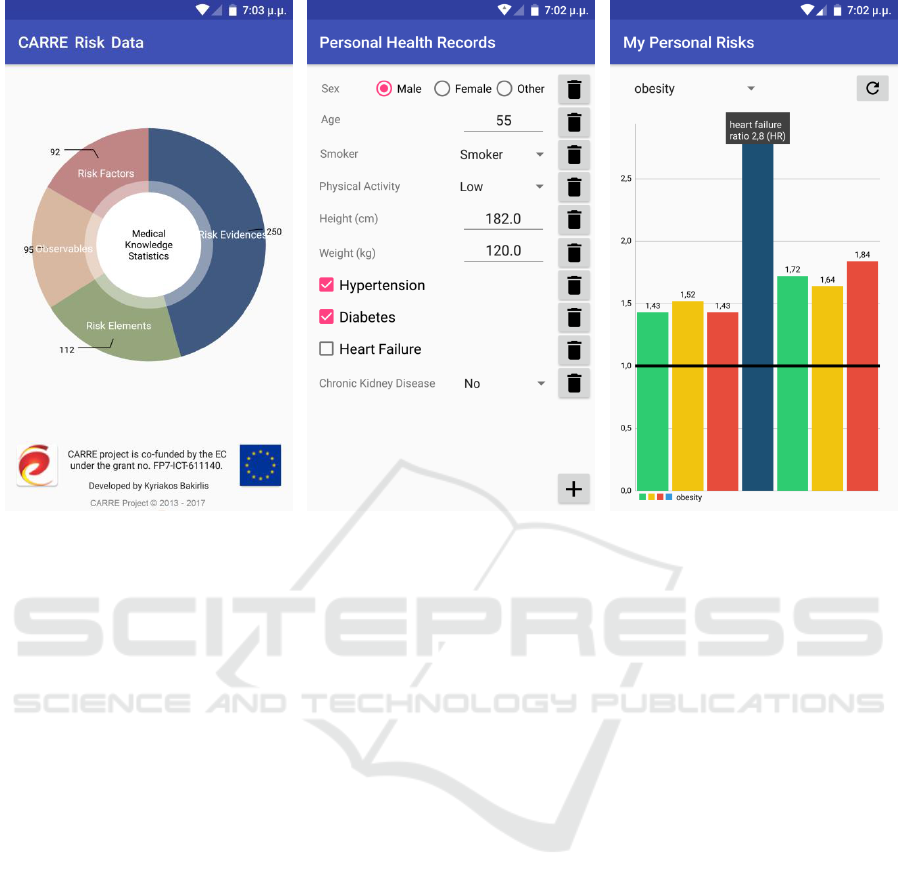
(a)
(b)
(c)
Figure 3: Screenshots of Risk Coach mobile Android application.
and browser; (b) create of a local copy personal
health related information, with fields dynamically
compiled based on what information is required to
customize available evidence on risk associations;
and (c) view and interact with personalized risk
information.
Indicative screenshots are shown in Figure 3.
Personalized risk visualization allows the user to
initially explore a summary of personal risk factors.
Each risk can then be expanded in a graph chart to
show all potential outcomes and the particular risk
ratio information. The user can also interact with
personal health information to set lifestyle goals and
observe the expected results in terms of risk
reduction; for example increase or decrease weight
to realize its impact on obesity related risk factors
4 DISCUSSION AND FURTHER
WORK
Patient empowerment is about designing and
delivering health and social care services in a way
that they are inclusive and enable citizens to take
control of their health care needs. According to the
European Network for Patient Empowerment
(ENOPE), the first mandate for an activated patient
is to be able to understand their health condition and
its effect on their body.
The Risk Coach application presented in this
paper addresses directly this basic requirement by
communicating state-of-the-art medical evidence on
common lifestyle related health risk determinants as
personalized to the individual. The application
enables the individual to have an overall view of
his/her own health status; understand potential
health deterioration and disease progression based
on current health status; and visually and
quantitatively investigate the impact each health
determinant may have on various health conditions.
Work in progress involves a randomized
controlled trial to access the efficacy of
communicating health risks to the general public via
the personalized application that preserves privacy
by design. The aim of the study is to assess user
satisfaction and efficacy of the application to
empower people and coach them towards a healthier
lifestyle. Primary objectives are to increase health
literacy, and increase level of empowerment.
Secondary objectives are to improve lifestyle habits
(smoking, physical activity, adherence to self-
monitoring and therapy) and test for application
acceptability and user satisfaction. The study
considers two different population groups: healthy
volunteers and chronic heart disease patients. The
HEALTHINF 2018 - 11th International Conference on Health Informatics
576

control arm will be given general written
information on common lifestyle related risks; the
intervention arm will be given access to the Risk
Coach mobile application.
Health literacy, before and after intervention,
will be assessed via the European Health Literacy
Questionnaire (Sørensen, 2013) and the Lipkus
Expanded Health Numeracy Scale (Lipkus, 2001).
Patient empowerment will be assessed via the
SUSTAINS instrument (Unver, 2013) which
considers three axes: enabling and strengthening
empowerment of patients; enabling better medical
results; and enabling a more efficient use of
healthcare resources and containing costs. Finally,
following the ISO standard ISO 9241 Part 11,
system usability will be measured by taking into
account the context of use of the system — i.e., who
is using the system, what they are using it for, and
the environment in which they are using it. Along
with an informative system evaluation, we will also
deploy the System Usability Score instrument
(Brooke, 1996).
Communicating health risks via interactive
personalized risk visualizations is expected to
increase health awareness, motivate persons to adopt
a healthier lifestyle, and contribute towards
increasing public health literacy and informed
shared decision making.
ACKNOWLEDGEMENTS
The work presented in this paper was partly
sponsored by the FP7-ICT project CARRE (Grant
No. 611140), funded in part by the European
Commission and Greek National Matching funds.
REFERENCES
Boon, N., Boyle, R., Bradbury, K., Buckley, J., Connolly,
S., Craig, S., ..., Wood, D., 2014. Joint British
Societies’ consensus recommendations for the
prevention of cardiovascular disease (JBS3), Heart,
100(Suppl 2), ii1-ii67.
Brooke, J., 1996. SUS: a “quick and dirty” usability scale.
Usability Evaluation in Industry, Taylor and Francis,
189-194.
Dingledine, R., Mathewson, N., Syverson, P., 2004. Tor:
the second-generation onion router. In Proc. of the
13th USENIX security symposium, 303-320.
Drosatos, G., Efraimidis, P.S., Williams, G., Kaldoudi, E.,
2016. Towards Privacy by Design in Personal e-
Health Systems. In Proc. Proceedings of the 9th
International Joint Conference on Biomedical
Engineering Systems and Technologies (BIOSTEC),
5(HEALTHINF), 472-477.
Efraimidis, P.S., Drosatos, G., Arampatzis, A.,
Stamatelatos, G., Athanasiadis, I., 2016. A privacy-by-
design contextual suggestion system for tourism.
Journal of Sensor and Actuator Networks, 5(2), 10.
Ernst & Young, 2012. Global Life Sciences Report,
Global Lifesciences Center Ltd.
European Heart Network AISB, 2017. European
cardiovascular disease statistics – 2017 Edition.
Guh, D. P., Zhang, W., Bansback, N., Amarsi, Z.,
Birmingham, C. L., Anis, A. H., 2009. The incidence
of co-morbidities related to obesity and overweight: a
systematic review and meta-analysis, BMC Public
Health, 9(1), 88.
Haveman-Nies, A., Tucker, K.L., de Groot, L.C.P.G.M.,
Wilson, P.W.F., Van Staveren, W.A., 2001.
Evaluation of dietary quality in relationship to
nutritional and lifestyle factors in elderly people of the
US Framingham Heart Study and the European
SENECA study. European Journal of Clinical
Nutrition, 55(10), 870.
Kizlaitis, R., Bileisiene, N., Puronaite, R., Stundys, D.,
Juozalenaite, G., Smagurauskas, T., Papazoglou, D.,
Zagkas, K., Roumeliotis, S., Passadakis, P., Pouliliou,
S., Drosatos, G., Makris, N., Kaldoudi, E., 2016.
Deliverable 7.4: Use case deployment & evaluation,
CARRE FP7-ICT project No. 611140, 1-117.
Knoops, K.T., de Groot, L.C., Kromhout, D., Perrin, A.E.,
Moreiras-Varela, O., Menotti, A., Van Staveren, W.
A., 2004. Mediterranean diet, lifestyle factors, and 10-
year mortality in elderly European men and women:
the HALE project. JAMA, 292(12), 1433-1439.
Kong, Y.M., Dahlke, C., Xiang, Q., Qian, Y., Karp, D.,
Scheuermann, R.H., 2011. Toward an ontology-based
framework for clinical research databases, J. Biomed.
Inform., 44(1), 48-58.
Lipkus, M., Samsa, G., Rimer, B.K., 2001. General perfor-
mance on a numeracy scale among highly educated
samples. Medical Decision Making, 21(1), 37-44.
Moreno, L.A., Gonzalez-Gross, M., Kersting, M., Molnar,
D., De Henauw, S., Beghin, L., ..., Ortega, F.B., 2008.
Assessing, understanding and modifying nutritional
status, eating habits and physical activity in European
adolescents: the HELENA Study. Public health
nutrition, 11(3), 288-299.
Perk, J., De Backer, G., Gohlke, H., Graham, I., Reiner,
Z., Verschuren, M., Albus, C., Benlian, P., Boysen, G.
et al., 2012. European Guidelines on cardiovascular
disease prevention in clinical practice, Eur Heart J.,
33(13), 1635-1701.
Romero-Tris, C., Viejo, A., Castellà-Roca, J., 2015. Multi-
party methods for privacy-preserving web search:
Survey and contributions. In Advanced Research in
Data Privacy. Springer International Publishing, 367-
387.
Sheridan, S., Pignone, M., Mulrow, C., 2003.
Framingham-based tools to calculate the global risk of
coronary heart disease. Journal of general internal
medicine, 18(12), 1039-1052.
Communicating Personalized Risk Factors for Lifestyle Coaching
577

Sim, I., Tu, S.W., Carini, S., Lehmann, H.P., Pollock, B.
H., Peleg, M., Wittkowski, K.M., 2014. The Ontology
of Clinical Research (OCRe): an informatics
foundation for the science of clinical research. Journal
of biomedical informatics, 52, 78-91.
Sørensen, K., Van den Broucke, S., Pelikan, J. M., Fullam,
J., Doyle, G., Slonska, Z., Kondilis, B., Stoffels, V.,
Osborne, R.H., Brand, H., 2013. Measuring health
literacy in populations: illuminating the design and
development process of the European Health Literacy
Survey Questionnaire (HLS-EU-Q), BMC public
health, 13(1), 948.
Spruijt-Metz, D., O’Reilly, G.A., Cook, L., Page, K.A.
Quinn, C., 2014. Behavioral contributions to the
pathogenesis of type 2 diabetes. Curr Diab Rep,
14(4), 475.
The Lancet Editor, 2012. Editorial: Patient Empower-
ment – who empowers whom?, The Lancet,
379(9827), 1677.
Third, A., Kaldoudi, E., Gkotsis, G., Roumeliotis, S.,
Pafili, K., Domingue, J., 2015. Capturing Scientific
Knowledge on Medical Risk Factors, In Proc. of the
15th International Semantic Web Conference (ISWC),
Springer International Publishing, 566-580.
Ünver, Ö., Atzori, W., 2013. Questionnaire for Patient
Empowerment Measurement Version 1.0 (Document
D3.2), SUSTAINS: Support USers To Access
INformation and Services, EU CT PSP Grant
Agreement No 29720, 1-74.
Woodward, M., Brindle, P., Tunstall-Pedoe, H., 2007.
Adding social deprivation and family history to
cardiovascular risk assessment: the ASSIGN score
from the Scottish Heart Health Extended Cohort
(SHHEC), Heart, 93(2), 172-176.
World Health Organization (WHO), 2012. Empowering
Patients, http://www.euro.who.int/en/what-we-do/
health-topics/noncommunicable-diseases/sections/
news/2012/4/empowering-patients, accessed on
5/10/2017.
World Health Organization (WHO), 2014. Global Status
Report on Non-communicable Diseases 2014,
http://www.who.int/nmh/publications/ncd-status-
report-2014/en/, accessed on 5/10/2017.
Zhao, Y., Parvinzamir, F., Wilson, S., Wei, H., Deng, Z.,
Portokallidis, N., Third, A., Drosatos, G., Liu, E.,
Dong, F., Marozas, V., Lukosevicius, A., Kaldoudi,
E., Clapworthy, G., 2017. Integrated Visualisation of
Wearable Sensor Data and Risk Models for
Individualised Health Monitoring and Risk
Assessment to Promote Patient Empowerment,
Journal of Visualisation, 20(2), 405-423.
HEALTHINF 2018 - 11th International Conference on Health Informatics
578
