
The Importance of Changes Observed in the Alternative Genetic Codes
Paweł Bła
˙
zej, Małgorzata Wnetrzak and Paweł Mackiewicz
Department of Genomics, Faculty of Biotechnology, University of Wrocław, F. Joliot-Curie 14a, 50-383 Wrocław, Poland
Keywords:
Alternative Genetic Code, Error Minimization, Genetic Code, Mutation.
Abstract:
The standard genetic code is a way of transmitting genetic information from DNA into protein world. The code
is universal for almost all living organisms on Earth but small deviations have been observed for many cellular
organelles and some specific groups of microorganisms with highly reduced genomes. Such modifications are
called alternative genetic codes. There is no consensus about the factors that caused or allowed these changes.
A popular concept assumes that the codes evolved under neutral evolution without adaptive constraints. In
this paper we present findings that argue with such view. We examined the level of error minimization in
amino acid replacements generated by the standard genetic code and its alternatives. We found that only
3 out of 23 tested alternative codes have worse quality than the standard genetic code. In agreement with
that, many single codon reassignments observed in the variants of the standard genetic code are generally
responsible for improving the quality of the codes under the studied criteria. These results indicate that the
codon reassignments observed in the existing alternative genetic codes could play an adaptive role in their
evolution to minimize translational and mutational errors. The study can help in designing alternative genetic
codes for artificially modified organisms in the framework of synthetic biology.
1 INTRODUCTION
The assumption about the universality of the standard
genetic code (SGC) was challenged by the discover-
ies of the genetic code variants (Osawa et al., 1992;
Jukes, 1996) especially because the SGC was initially
considered a ’frozen accident’ (Crick, 1968). These
deviations are mainly observed in the codes operat-
ing in organelles, especially in mitochondria (Osawa
et al., 1989; Crozier and Crozier, 1993; Boore and
Brown, 1994). The alternative codes are also involved
in translation of proteins coded in nuclear genomes of
various unicellular eukryotes (Schneider et al., 1989;
Sanchez-Silva et al., 2003) and some bacteria, espe-
cially in parasites and symbionts with highly reduced
genomes (Lim and Sears, 1992; McCutcheon et al.,
2009; Campbell et al., 2013). In recent years, the
number of newly discovered alternative genetic codes
has significantly increased (Heaphy et al., 2016; Za-
honova et al., 2016). This quite large set of alternative
codes is a good starting point to analyze the proper-
ties and the potential evolutionary tendencies of these
codes and the SGC.
There are three main types of deviations from the
standard genetic code found in its alternatives: (i) re-
assignments of codons coding for typical 20 amino
acids and stop translation signal, (ii) loss of codon
assignments resulting in the occurrence of unused
codons, (iii) incorporation of new amino acids, e.g.
selenocysteine and pyrrolysine. These three types of
changes were discussed and modeled by (Sengupta
et al., 2007). They result usually from mutations and
the editing of tRNA genes or the posttranscriptional
modifications of bases in tRNA molecules (Santos
et al., 2004). However, it is not clear how and why
these changes occurred and were fixed. It is gener-
ally believed that they evolved neutrally without any
adaptive pressure through genetic drift and mutational
pressure that drove small populations and their tiny
genomes toward the high AT-content (Freeland et al.,
2000; Koonin, 2017). Such changes can influence
codon usage and, in extreme cases, can lead to dis-
appearance of GC-containing codons (Santos et al.,
2004). Alternatively, the variants of the SGC could
have evolved to reduce protein synthesis costs (Swire
et al., 2005) or to minimize effects of point muta-
tions; such properties were observed in some of these
codes (Kurnaz et al., 2010; Morgens and Cavalcanti,
2013). Here we focused on the latter aspects of the
genetic code evolution and analyzed the optimality of
many genetic code variants to assess their robustness
in terms of amino acid replacements.
154
Bła
˙
zej, P., Wnetrzak, M. and Mackiewicz, P.
The Importance of Changes Observed in the Alternative Genetic Codes.
DOI: 10.5220/0006642001540159
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 3: BIOINFORMATICS, pages 154-159
ISBN: 978-989-758-280-6
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2 METHODS
The examined alternative genetic codes were
downloaded from the NCBI taxonomy web page:
www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi.
From the whole set of 30 available genetic code
variants we chose those which differed in codon as-
signments from the standard genetic code, including
also the codes with ambiguous codon assignments.
In total, we studied 23 alternative codes (Table 1).
Table 1: The genetic code variants studied in this work
with the number of codon reassignments. In the case of the
genetic codes with ambiguous reassignments, we included
two different versions of their structures: with all possible
codon assignments (all) and with only unambiguous assign-
ments (unamb).
Genetic code name Num. of reassig.
Alternative Flatworm Mitochondrial Code 5
Trematode Mitochondrial Code 5
Invertebrate Mitochondrial Code 4
Flatworm Mitochondrial Code 4
Ascidian Mitochondrial Code 4
Vetebrate Mitochondrial Code 4
Candylostoma Nuclear Code (all) 3
Karyorelict Nuclear Code (all) 3
Blastocrithidia Nuclear Code (all) 3
Pterobranchia Mitochondrial Code 3
Hexamita Nuclear Code 2
Karyorelict Nuclear Code (unamb) 2
Mesodium Nuclear Code 2
Peritrich Nuclear Code 2
Scenedesmus Mitochondrial Code 2
Gracilibacteria Code 1
Euploid Nuclear Code 1
Blastocrithidia Nuclear Code (unamb) 1
Protozoan Mitochondrial Code 1
Chlorophycean Mitochondrial Code 1
Tannophilus Nuclear Code 1
Alternative Yeast Nuclear Code 1
Thraustochytrium Mitochondrial Code 1
To test the optimality of a given code, we had to use
a specific measure describing costs of amino acid re-
placements. In this work, for a given genetic code
(code) we used the following cost measure:
F(code) =
∑
<i, j>∈D
[ f (i) − f ( j)]
2
, (1)
where D is the set of pairs of codons that differ in
one nucleotide substitution, whereas f (i) and f ( j) are
the polarity values of the amino acids (Woese, 1973)
coded by the codons i and j, respectively. There-
fore, the measure F(code) represents the total sum of
the squared differences between polarity properties of
amino acids for the codon pairs differing in one sub-
stitution. The main reason for using the polarity prop-
erty to evaluate the cost of a genetic code follows from
the fact that this characteristic is independent of the
specificity of the SGC structure and was commonly
applied in testing the optimality of the standard ge-
netic code (Di Giulio, 1989; Haig and Hurst, 1991;
Freeland and Hurst, 1998; Santos and Monteagudo,
2010; Bła
˙
zej et al., 2016).
All the single nucleotide substitutions that lead
to nonsense mutations, i.e. to the replacement of an
amino acid by a stop translation codon, were included
in the calculation as the maximum of squared differ-
ences computed for any possible pair of amino acids.
One could argue with this assumption. However, it
is known that the nonsense mutations are very dele-
terious because they result in incomplete and usually
nonfunctional proteins. Therefore, it seems reason-
able to assume such large costs for this type of substi-
tution.
Furthermore, we calculated three characteristics
in the case of the genetic codes with ambiguous
codon assignments. F(code all) included all possi-
ble codon assignments and F(code unamb) included
only unambiguous codon assignments. Additionally,
we calculated the arithmetic mean F(code mean)
from F(code all) and F(code unamb). As a re-
sult, F(code mean) assumes that ambiguous codon
assignments occur with equal probability.
To validate the properties of the genetic codes we
compare them with all possible 1240 theoretical codes
that differed from the SGC by one codon assignment.
3 RESULTS
3.1 Optimality of the Standard and
Alternative Genetic Codes
The comparison of the cost function F calculated for
the standard genetic code and its alternatives showed
that the SGC is not the best optimized code, in
terms of the polarity property, similarly to the re-
sults obtained by (Morgens and Cavalcanti, 2013).
Only 3 out of 23 considered alternative genetic codes
have greater (i.e. worse) F value than the SGC, i.e.
F(SGC) = 5641.46 (Figure 1):
1. Vetebrate Mitochondrial Code: F(code) =
6716.48;
2. Alternative Yeast Nuclear Code: F(code) =
5651.86;
3. Thraustochytrium Mitochondrial Code:
F(code) = 6283.02.
The best found code according to the polarity
costs is Karyorelict Nuclear Code including all am-
The Importance of Changes Observed in the Alternative Genetic Codes
155

●
●
●
●
●
●
●●
●
●
●●
●
●
●
●
●
●●
●
●
●
●
1 2 3 4 5
3000 4000 5000 6000
number of reassignments
cost value
Figure 1: The cost values (black circles) calculated for alter-
native genetic codes. They are compared with the number
of codon reassignments (x-axis) and also with the cost of
the standard genetic code (the bold horizontal line). It is
visible that many alternative codes lie below the horizontal
line and are better optimized to minimize the costs of amino
acid replacements than the SGC.
biguous reassignments of codons. It reaches the min-
imum cost value F(code) = 2945.12 which is nearly
two times lower than F(SGC). Similarly, there are
theoretical genetic codes with one codon reassign-
ment that have smaller cost values than the SGC.
The best one of them has the cost value F(code) =
4766.28. These results suggest that the standard ge-
netic code can be significantly improved even by a
small number of codon reassignments.
On the other hand, there is a quite large probabil-
ity to deteriorate the SGC structure in terms of the F
value just by one random reassignment because more
than 330 theoretical codes out of all 1240 considered,
i.e. 27% have lower cost values than the SGC (Figure
2).
3.2 The Properties of Codon
Assignments in Alternative Genetic
Codes
It is interesting to examine the features of the alter-
native genetic codes which are better optimized than
the SGC. First we calculated the number of occur-
rences of all individual codon reassignments observed
in the alternative genetic codes under study (Table 3).
It is evident that these changes can be classified into
three groups (Figure 3). The first one (A) contains
all codon reassignments from stop translation signal
Table 2: The cost values F calculated for all genetic code
variants studied in this work in comparison to the the stan-
dard genetic code (SGC) and the best theoretical genetic
code with single codon reassignment. We included three
different characteristics of the genetic code variants: with
all possible codon assignments (all) and with only unam-
biguous assignment (unamb) and the average value (mean)
of the cost value.
genetic code name cost
Candylostoma Nuclear Code (all) 2945.12
Karyorelict Nuclear Code (all) 2945.12
Karyorelict Nuclear Code (mean) 3484
Blastocrithidia Nuclear Code (all) 3697.04
Alternative Flatworm Mitochondrial Code 3942.66
Hexamita Nuclear Code 4022.88
Karyorelict Nuclear Code (unamb) 4022.88
Mesodium Nuclear Code 4116.26
Blastocrithidia Nuclear Code (mean) 4250.7
Candylostoma Nuclear Code (mean) 4293.29
Peritrich Nuclear Code 4692.9
Trematode Mitochondrial Code 4692.92
Invertebrate Mitochondrial Code 4696.84
Flatworm Mitochondrial Code 4706.84
Ascidian Mitochondrial Code 4748.84
the best theoret. code with one reassign. 4766.28
Gracilibacteria Code 4783.56
Euploid Nuclear Code 4795.08
Blastocrithidia Nuclear Code (unamb) 4804.36
Protozoan Mitochondrial Code 4804.36
Pterobranchia Mitochondrial Code 4839.88
Chlorophycean Mitochondrial Code 4936.3
Scenedesmus Mitochondrial Code 5575.14
Tannophilus Nuclear Code 5630.96
the standard genetic code 5641.46
Alternative Yeast Nuclear Code 5651.86
Thraustochytrium Mitochondrial Code 6283.02
Vetebrate Mitochondrial Code 6716.48
to one of the 20 standard amino acids. There are 31
such changes which make over 55% of all possible
56 codon reassignments found in the studied alterna-
tive genetic codes. The second group (B) includes in
total 21 codon reassignments which are involved in
changing encoded amino acids. Finally, we have only
4 cases in which codons originally encoding amino
acids change their meaning to the stop translation sig-
nal (the group C).
It should be noted that the codon reassignments
belonging to the first and, at the same time, the largest
group (Table 3) are the most desired in terms of mini-
mizing the F value because each of these changes de-
creases the cost value in comparison with the F(SGC)
(Figure 3). For example, the single assignment of stop
codon TGA to tryptophane, which is the most fre-
quently observed change, i.e. 12 times, diminishes the
cost of the F(SGC) = 5641.46 to F(T GA → Trp) =
4804.36. This reassignment is one of the best ones
because the resulting cost for the code differs by less
BIOINFORMATICS 2018 - 9th International Conference on Bioinformatics Models, Methods and Algorithms
156
5000 5500 6000 6500
0.000 0.001 0.002 0.003 0.004 0.005
cost value
Density
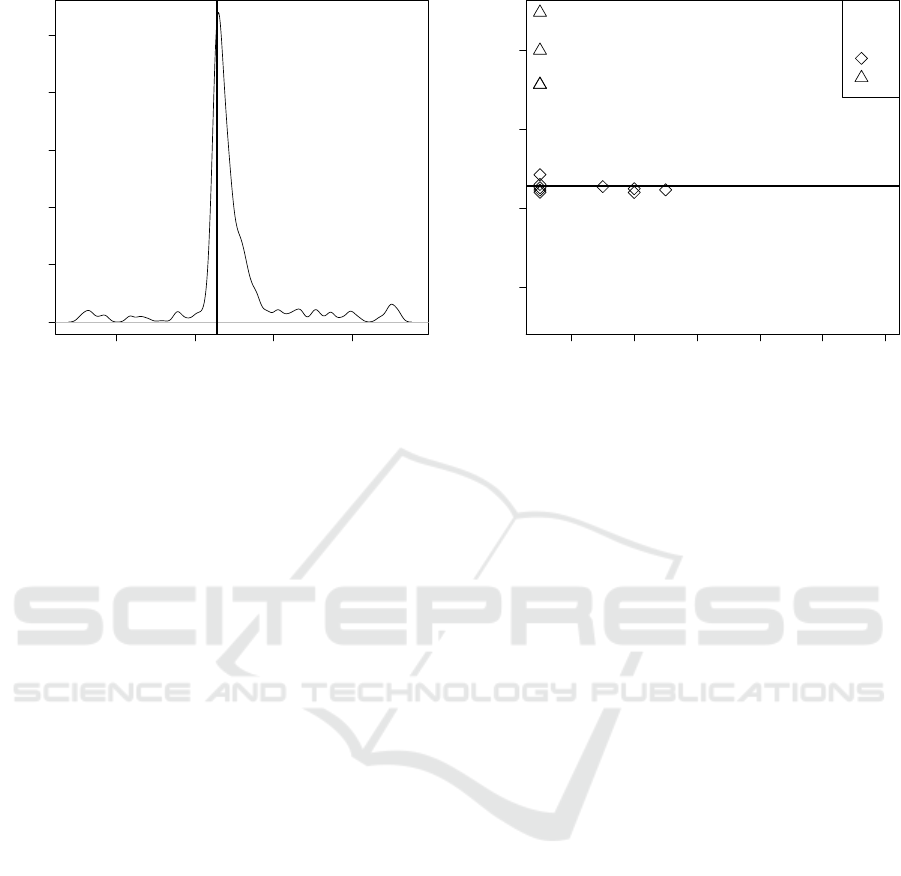
Figure 2: The density plot of the frequency of cost values F
calculated for the theoretical genetic codes that differ from
the standard genetic code in one codon assignment. The
cost value calculated for the standard genetic code F(SGC)
is marked by the vertical bold line. It is evident that F(SGC)
value is situated closer to smaller values. Thereby, it is less
probable to generate at random a code better than the SGC
just by one codon reassignment.
than 1% from the best possible cost obtained by the
single change F(T GA → Ala) = 4766.28.
The reassignments in the second group have a
rather small influence on the cost values in compar-
ison with the change of codon’s meaning from a stop
translation signal to an amino acid (Figure 3). How-
ever, many of these missense reassignments can also
improve, although slightly, the F value in comparison
with F(SGC), i.e. F(AGG → Ser) = 5601.14. The
reassignments that decrease the F value are more fre-
quent in the studied alternative genetic codes than the
reassignments increasing the costs of amino acid re-
placement, e.g. F(AGG → Lys) = 5713.46. The for-
mer were found in 21 cases, whereas the latter in only
four cases.
The third group of reassignments contain codons
that formerly coded for an amino acid but then
changed their meaning into the stop translation sig-
nal. Such changes have the most dramatic impact on
the cost value and encoded proteins. Generally, they
are responsible for the significant increase (over 10%)
of the F value in comparison with F(SGC) (Figure 3).
However, they were observed only in four alternative
genetic codes (Table 3).
2 4 6 8 10 12
5000 5500 6000 6500
number of occurences
cost value
●
●
●
●
●
●
●
●
●
●
●
●
groups
A
B
C
Figure 3: The relationship between the cost values and the
number of occurrences of individual codon reassignments
in the studied alternative genetic codes. The reassignments
were classified into three groups where the codon’s mean-
ing is changed: from a stop translation signal to an amino
acid (A), from one amino acid to another (B) and from an
amino acid to a stop translation signal (C). The results are
compared with the cost value calculated for the standard ge-
netic code (the horizontal bold line).
4 DISCUSSION
Our study on the optimality of the alternative genetic
codes in comparison to the standard genetic code
showed that many of these variants contain codon re-
assignments that decrease the costs of amino acid re-
placements described by the polarity values. It im-
plies that the alternatives did not necessarily originate
as a result of the neutral evolution but they could have
evolved under adaptational factors and at least some
of their codon reassignments were favored by the se-
lection (Kurnaz et al., 2010). Such reorganizations of
the code could have occured in small populations with
tiny genomes, in which the changes did not influence
the large number of encoded proteins.
The SGC is, however, less optimal in compari-
son to most of its alternatives. This finding does not
fully support the adaptive hypothesis postulating that
the code structure evolved to minimize the effects
of amino acid replacements and errors during trans-
lation of proteins (Epstein, 1966; Haig and Hurst,
1991; Freeland et al., 2003; Goodarzi et al., 2005).
This concept is attractive but the deleterious effects of
mutations on protein properties can be minimized by
other mechanisms, i.e. the direct optimization of the
The Importance of Changes Observed in the Alternative Genetic Codes
157
Table 3: The number of occurrences of single codon reas-
signments observed in the studied alternative genetic codes.
The table includes also the costs calculated for genetic
codes with exactly one of such substitutions and the type
of reassignment used in Figure 3.
Codon In SGC In altern. Cost Occur. Type
TGA Stp Trp 4804.36 12 A
AGA Arg Ser 5617.78 5 B
TAA Stp Gln 5083.72 4 A
TAG Stp Gln 4843.06 4 A
ATA Ile Met 5624.82 4 B
AGG Arg Ser 5601.14 4 B
AAA Lys Asn 5638.02 3 B
TAA Stp Glu 5377.78 2 A
TAG Stp Glu 5219.02 2 A
TAG Stp Leu 4936.3 2 A
CTG Leu Ala 5630.96 1 B
TGA Stp Cys 4795.08 1 A
AGA Arg Gly 5616.02 1 B
AGG Arg Gly 5602.58 1 B
TGA Stp Gly 4783.56 1 A
AGG Arg Lys 5713.46 1 B
CTG Leu Ser 5651.86 1 B
AGA Arg Stp 6737.06 1 C
AGG Arg Stp 6497.1 1 C
TTA Leu Stp 6283.02 1 C
TCA Ser Stp 6280.3 1 C
TAA Stp Trp 5167.54 1 A
TAA Stp Tyr 5141.14 1 A
TAG Stp Tyr 4879.02 1 A
mutational rate and pattern on the fixed genetic code
(Bła
˙
zej et al., 2015; Bła
˙
zej et al., 2017). There-
fore, the main role of the assignments of amino acids
to codons in the SGC could have been played by
the expansion of biosynthetic pathways and the step-
wise addition of newly synthesized amino acids into
the code, according to the co-evolution hypothesis
(Wong, 1975; Di Giulio, 1999; Wong et al., 2016;
Di Giulio, 2017). Under this scenario, the present
structure of SGC evolved from an ancestral version
including a smaller number of simple amino acids
that were at the beginning of the biosynthetic path-
ways. Next, other amino acids were incorporated
into the code when more complex metabolic networks
evolved. The newly synthesized amino acids took
over the codons of their precursors.
The idea of studying the properties of the genetic
code variants seems very promising in the light of de-
signing alternative versions of the code for artificially
modified organisms (Xie and Schultz, 2006; Chin,
2014). Such modifications can lead to production of
peptides or proteins including unnatural amino acids
and showing enhanced or novel properties. The intro-
duced codon reassignments can also help to test the
protein structure and function in global scale. More-
over, the knowledge about the optimality of the ge-
netic codes may enable us to construct new artifi-
cial organisms in the framework of synthetic biology.
Such organisms could be characterized by for exam-
ple a higher fidelity of the protein synthesis and a
higher resistance to the mutations causing amino acid
replacements.
ACKNOWLEDGMENTS
This work was supported by the National Science
Centre Poland (Narodowe Centrum Nauki, Polska)
under Grant Miniatura no. 2017/01/X/NZ2/00608.
REFERENCES
Bła
˙
zej, P., Mackiewicz, D., Grabinska, M., Wnetrzak, M.,
and Mackiewicz, P. (2017). Optimization of amino
acid replacement costs by mutational pressure in bac-
terial genomes. Scientific Reports, 7:1061.
Bła
˙
zej, P., Miasojedow, B., Grabinska, M., and Mack-
iewicz, P. (2015). Optimization of mutation pressure
in relation to properties of protein-coding sequences
in bacterial genomes. PloS One, 10:e0130411.
Bła
˙
zej, P., Wnetrzak, M., and Mackiewicz, P. (2016). The
role of crossover operator in evolutionary-based ap-
proach to the problem of genetic code optimization.
Biosystems, 150:61–72.
Boore, J. L. and Brown, W. M. (1994). Complete dna se-
quence of the mitochondrial genome of the black chi-
ton, katharina tunicata. Genetics, 138(2):423–43.
Campbell, J. H., O’Donoghue, P., Campbell, A. G.,
Schwientek, P., Sczyrba, A., Woyke, T., Sll, D., and
Podar, M. (2013). UGA is an additional glycine codon
in uncultured SR1 bacteria from the human micro-
biota. Proc Natl Acad Sci U S A, 110:5540–5545.
Chin, J. W. (2014). Expanding and reprogramming the ge-
netic code of cells and animals. Annu Rev Biochem,
83:379–408.
Crick, F. H. (1968). The origin of the genetic code. J Mol
Biol, 38(3):367–79.
Crozier, R. H. and Crozier, Y. C. (1993). The mitochon-
drial genome of the honeybee Apis mellifera: com-
plete sequence and genome organization. Genetics,
133(1):97–117.
Di Giulio, M. (1989). The extension reached by the mini-
mization of the polarity distances during the evolution
of the genetic code. J Mol Evol, 29(4):288–93.
Di Giulio, M. (1999). The coevolution theory of the origin
of the genetic code. J Mol Evol, 48(3):253–5.
Di Giulio, M. (2017). Some pungent arguments against
the physico-chemical theories of the origin of the ge-
netic code and corroborating the coevolution theory. J
Theor Biol, 414:1–4.
Epstein, C. J. (1966). Role of the amino-acid ”code” and of
selection for conformation in the evolution of proteins.
Nature, 210(5031):25–8.
BIOINFORMATICS 2018 - 9th International Conference on Bioinformatics Models, Methods and Algorithms
158

Freeland, S. J. and Hurst, L. D. (1998). The genetic code is
one in a million. J Mol Evol, 47(3):238–248.
Freeland, S. J., Knight, R. D., Landweber, L. F., and Hurst,
L. D. (2000). Early fixation of an optimal genetic
code. Mol Biol Evol, 17(4):511–8.
Freeland, S. J., Wu, T., and Keulmann, N. (2003). The
case for an error minimizing standard genetic code.
Origins of Life and Evolution of the Biosphere, 33(4-
5):457–477.
Goodarzi, H., Najafabadi, H. S., Hassani, K., Nejad, H. A.,
and Torabi, N. (2005). On the optimality of the ge-
netic code, with the consideration of coevolution the-
ory by comparison of prominent cost measure matri-
ces. J Theor Biol, 235(3):318–25.
Haig, D. and Hurst, L. D. (1991). A quantitative measure
of error minimization in the genetic-code. J Mol Evol,
33(5):412–417.
Heaphy, S. M., Mariotti, M., Gladyshev, V. N., Atkins, J. F.,
and Baranov, P. V. (2016). Novel ciliate genetic code
variants including the reassignment of all three stop
codons to sense codons in Condylostoma magnum.
Mol Biol Evol, 33:2885–2889.
Jukes, T. H. (1996). Neutral changes and modifications
of the genetic code. Theoretical Population Biology,
49(2):143–145.
Koonin, E. V. (2017). Frozen accident pushing 50: Stere-
ochemistry, expansion, and chance in the evolution of
the genetic code. Life (Basel), 7(2).
Kurnaz, M. L., Bilgin, T., and Kurnaz, I. A. (2010). Certain
non-standard coding tables appear to be more robust
to error than the standard genetic code. J Mol Evol,
70(1):13–28.
Lim, P. O. and Sears, B. B. (1992). Evolutionary relation-
ships of a plant-pathogenic mycoplasmalike organism
and Acholeplasma laidlawii deduced from two riboso-
mal protein gene sequences. J. Bacteriol, 174:2606–
2611.
McCutcheon, J. P., McDonald, B. R., and Moran, N. A.
(2009). Origin of an alternative genetic code in the
extremely small and GC-rich genome of a bacterial
symbiont. Plos Genetics, 5(7).
Morgens, D. W. and Cavalcanti, A. R. (2013). An alterna-
tive look at code evolution: using non-canonical codes
to evaluate adaptive and historic models for the origin
of the genetic code. J Mol Evol, 76(1-2):71–80.
Osawa, S., Jukes, T. H., Watanabe, K., and Muto, A. (1992).
Recent evidence for evolution of the genetic code. Mi-
crobiol Rev, 56(1):229–64.
Osawa, S., Ohama, T., Jukes, T. H., and Watanabe, K.
(1989). Evolution of the mitochondrial genetic code.
I. origin of AGR serine and stop codons in metazoan
mitochondria. J Mol Evol, 29(3):202–7.
Sanchez-Silva, R., Villalobo, E., Morin, L., and Torres, A.
(2003). A new noncanonical nuclear genetic code:
translation of UAA into glutamate. Curr Biol, 13:442–
447.
Santos, J. and Monteagudo, A. (2010). Study of the genetic
code adaptability by means of a genetic algorithm. J
Theor Biol, 264(3):854–865.
Santos, M. A., Moura, G., Massey, S. E., and Tuite, M. F.
(2004). Driving change: the evolution of alternative
genetic codes. Trends Genet, 20(2):95–102.
Schneider, S. U., Leible, M. B., and Yang, X. P.
(1989). Strong homology between the small subunit
of ribulose-1,5-bisphosphate carboxylase/oxygenase
of two species of Acetabularia and the occurrence of
unusual codon usage. Mol Gen Genet, 218:445–452.
Sengupta, S., Yang, X., and Higgs, P. G. (2007). The mech-
anisms of codon reassignments in mitochondrial ge-
netic codes. J Mol Evol, 64(6):662–88.
Swire, J., Judson, O. P., and Burt, A. (2005). Mitochon-
drial genetic codes evolve to match amino acid re-
quirements of proteins. J Mol Evol, 60(1):128–39.
Woese, C. R. (1973). Evolution of the genetic code. Natur-
wissenschaften, 60(10):447–59.
Wong, J. T. (1975). A co-evolution theory of the genetic
code. Proc Natl Acad Sci U S A, 72(5):1909–12.
Wong, J. T., Ng, S. K., Mat, W. K., Hu, T., and Xue, H.
(2016). Coevolution theory of the genetic code at age
forty: Pathway to translation and synthetic life. Life
(Basel), 6(1).
Xie, J. M. and Schultz, P. G. (2006). Innovation: A chemical
toolkit for proteins - an expanded genetic code. Nat
Rev Mol Cell Biol, 7(10):775–782.
Zahonova, K., Kostygov, A. Y., Sevcikova, T., Yurchenko,
V., and Elias, M. (2016). An unprecedented non-
canonical nuclear genetic code with all three termi-
nation codons reassigned as sense codons. Curr Biol,
26:2364–2369.
The Importance of Changes Observed in the Alternative Genetic Codes
159
