
LIBS based Tissue Differentiation for Er:YAG Surgical Laser
Fanuel Mehari
1,2
, Benjamin Lengenfelder
1,2
, Robert Figura
1
, Florian Kl
¨
ampfl
1
and Michael Schmidt
1,2
1
Friedrich-Alexander-Universit
¨
at Erlangen-N
¨
urnberg (FAU), Institute of Photonic Technologies (LPT),
Konrad-Zuse-Straße 3/5, 91052 Erlangen, Germany
2
Erlangen Graduate School in Advanced Optical Technologies (SAOT), Paul-Gordan-Straße 6, 91052 Erlangen, Germany
Keywords:
LIBS (Laser-induced Breakdown Spectroscopy), Biophotonics, Laser Surgery, Tissue Differentiation, Er:YAG
Laser.
Abstract:
The analytical technique laser-induced breakdown spectroscopy (LIBS) is becoming an attractive technique in
the field of medicine. One emerging application is the differentiation of biological tissues in real-time during
laser surgery. This work attempts to further investigate the use of LIBS together with a surgical Er:YAG laser.
The main goal here is to investigate the effect of body fluids as potential contaminants during LIBS based
differentiation of soft tissues. Furthermore, the work attempts to exploit the use of the surgical laser as a
cleaning laser and compares the effect when only the LIBS laser is used for tissue differentiation. The study
shows that body fluids have a significant influence on the LIBS spectra and that a surgical laser might serve as
an in-vivo cleaner leading to improved tissue classification during laser surgery.
1 INTRODUCTION
Lasers have become one of the most important tools
used in modern medicine. Due to the unique in-
trinsic properties of laser light, such as monochro-
maticity, focusability and high intensity, lasers are
being used in general surgery (Walter, 1999) as well
as in specialized disciplines like dermatology (Gold-
berg., 2013), and ophtalmology (Solomon, 2009).
One of the most common lasers used as laser scalpel
is the Er:YAG since it offers very high absorption
in water and thus efficient processing for all tissue
types. The advantages laser surgery brings about
include providing a sterile surgery technique along
with highly precise treatment areas with little to no
thermic damage. However, the laser scalpel lacks any
feedback including tactile information and it becomes
difficult to operate without inflicting damage to crit-
ical tissues such as muscle or nerves. This problem
leads to the investigation of various feedback mod-
alities providing tissue discrimination for laser sur-
gery. As a consequence, optical based techniques
for tissue discrimination have gained significant in-
terest. One of the optical techniques that is drawing
attention for a feedback system is LIBS. LIBS uses
a pulsed laser to generate a plasma plume which va-
porizes a small volume of a given specimen. Using
a spectrometer, the plasma decay emission spectrum
is analyzed and then used to identify and quantify the
constituent elements the specimen is made of (Cre-
mers and Radziemski., 2013). LIBS has been suc-
cessfully used to analyze various kinds of tissues un-
der ex-vivo conditions, including biological tissues
such as bone and cartilage (Mehari, 2014). Neverthe-
less, the fact that in-vivo conditions profoundly dif-
fer from those outside the body has to be taken into
consideration. Tissue surfaces inside the body can be
contaminated with body fluids containing various ele-
ments that could have an influence on the obtained
LIBS spectra.
2 OBJECTIVES
In this work, the influence of simulated in-vivo con-
ditions (by putting a thin saline layer on top of the tis-
sue samples) on the LIBS classification is first invest-
igated without the usage of a surgical laser (single-
pulse). In addition, it considers the use of a con-
ventional surgical Er:YAG laser as a cleaning instru-
ment to free the tissue’s surface from liquid contam-
inants prior to LIBS analysis as one possible way to
minimize the influence of body fluids (double-pulse).
First, the LIBS spectra of contaminated and dry fat
and muscle tissue is evaluated without the usage of
Mehari, F., Lengenfelder, B., Figura, R., Klämpfl, F. and Schmidt, M.
LIBS based Tissue Differentiation for Er:YAG Surgical Laser.
DOI: 10.5220/0006637402470251
In Proceedings of the 6th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2018), pages 247-251
ISBN: 978-989-758-286-8
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
247
a Er:YAG laser. Second, a conventional surgical
Er:YAG laser is used in combination with LIBS ana-
lysis for contaminated and dry fat and muscle tissue.
3 MATERIALS AND METHODS
3.1 Single-pulse LIBS
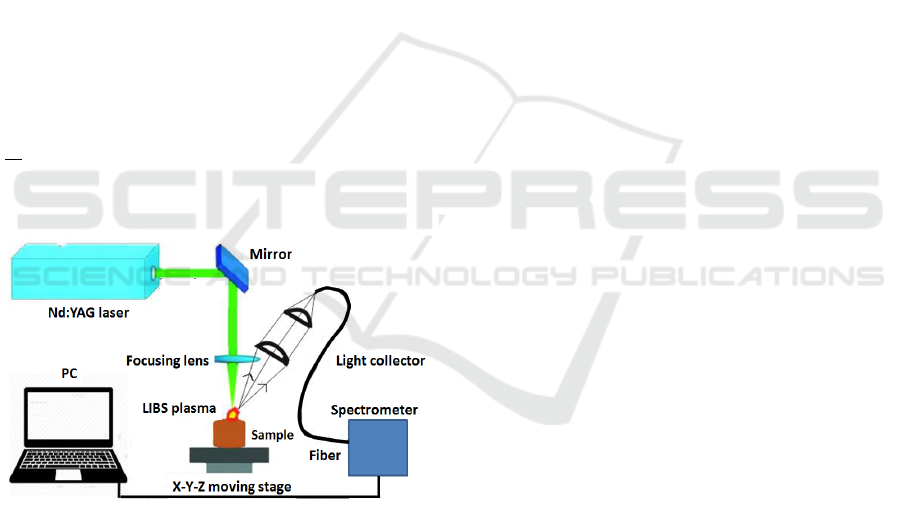
Figure 1 shows a schematic overview of the setup
used for single-pulse. The experimental setup essen-
tially consists of three major components and sev-
eral auxiliary devices. The centerpiece is a Nd:YAG
(532 nm) laser whose laser beam is focused onto the
tissue samples. Light signals from the LIBS plasma
are collected using an array of convex lenses and
an optical fiber cable that is connected to a spectro-
meter for detecting and analyzing the signals. The
array of convex lenses for LIBS signal collection
is focused onto the tissue surface and aligned with
the laser focus. For signal transport to the spectro-
meter a multimode UV grade fiber cable was used.
Spectral analysis is performed with an Echelle spec-
trograph (Andor Mechelle 5000) of resolving power
λ
∆λ
= 6000. The tissue samples were placed in a petri
dish that was put on a XYZ-translation stage to be
able to move around the sample in 3D.
Figure 1: Optical setup for single-pulse LIBS.
3.2 Double-pulse LIBS
An adjusted setup is implemented for LIBS experi-
ments following a laser pulse of a surgical Er:YAG
laser (2.94 µm, Glissando, WaveLight(T M)), Erlan-
gen, Germany). The Nd:YAG laser in the single-pulse
experiments was used. The Er:YAG laser is operated
at a repetition rate of 10 Hz with a pulse duration of
350 µs and a pulse energy of 200 mJ. To use it for
LIBS experiments in this study, the laser head is fixed
steady next to the moving stage and directed onto the
tissue sample in a 45
◦
angle. The Er:YAG laser’s fo-
cus lies slightly above the sample surface. Moreover,
it is aligned with the focus of the Nd:YAG laser to
ensure that both lasers hit the same area on the tissue
surface for one measurement. In order to synchron-
ize the two lasers, a pulse detector for infrared light
(DoroTek Lab Bench Detector, 2 − 12µm) is used to
detect the reflected light of the Er:YAG laser beam in-
cident on the sample’s surface. Similarly to the first
setup, this signal is then used to trigger the pulse gen-
erator. Subsequently, the pulse generator triggers the
Nd:YAG laser with a temporal delay of 100 µs. This
delay combined with an internal processing delay of
approximately 300 µs from flashlamp triggering to Q-
switch activation, ensures that the Nd:YAG laser al-
ways fires after the Er:YAG laser’s pulse has ceased.
3.3 Sample Preparation and Data
Analysis
Fat and muscle tissue samples were extracted from
bisected ex-vivo pig heads at the Department of Oral
and Maxillofacial Surgery University Hospital Erlan-
gen. Using a knife, the tissues were cut into nearly
rectangular pieces of about 5 − 8 mm thickness for
LIBS measurements.
An isotonic saline solution (9 g NaCl per liter) is used
to create a thin liquid layer on the tissue surface to
partly mimic a layer of body fluids present at in-vivo
conditions, for example, during general surgery. Us-
ing a small medical syringe, a few droplets of sa-
line solution are applied onto the tissue surface to be
able to spread the liquid in such a manner to establish
a consistent liquid layer thickness of approximately
20 − 30µm (measured by OCT) that stays steady over
the course of the double-pulse LIBS experiments.
For the LIBS experiments, 6 tissue samples of each,
muscle and fat tissue, are measured in both LIBS
setups. Each tissue sample is measured 100 times,
where one half of the measurement set is obtained
under dry conditions and the other one with a sa-
line solution. Therefore, 600 LIBS measurements are
taken in each of the experimental setups.
The experimental data obtained from LIBS measure-
ments of the tissue samples is subsequently prepared
to undergo different statistical analysis techniques to
investigate the effect of the saline solution and the
cleaning effect of the surgical laser. Here, statistical
analysis is performed to observe the similarity and
differences among the tissues investigated under dif-
ferent conditions. As a first step, Principal Compon-
ent Analysis (PCA) is used to reduce the high dimen-
sionality of the data before Linear Discriminant Ana-
lysis (LDA) is performed on the data to classify each
PHOTOPTICS 2018 - 6th International Conference on Photonics, Optics and Laser Technology
248
data set into groups. As a final step, the method of
Receiver Operating Characteristics (ROC) determines
the performance of the classification by LDA. Import-
ant values for performance assessment of ROC ana-
lysis are the sensitivity (true positive rate), specificity
(false negative rate).
4 RESULTS AND DISCUSSION
4.1 Single-pulse LIBS
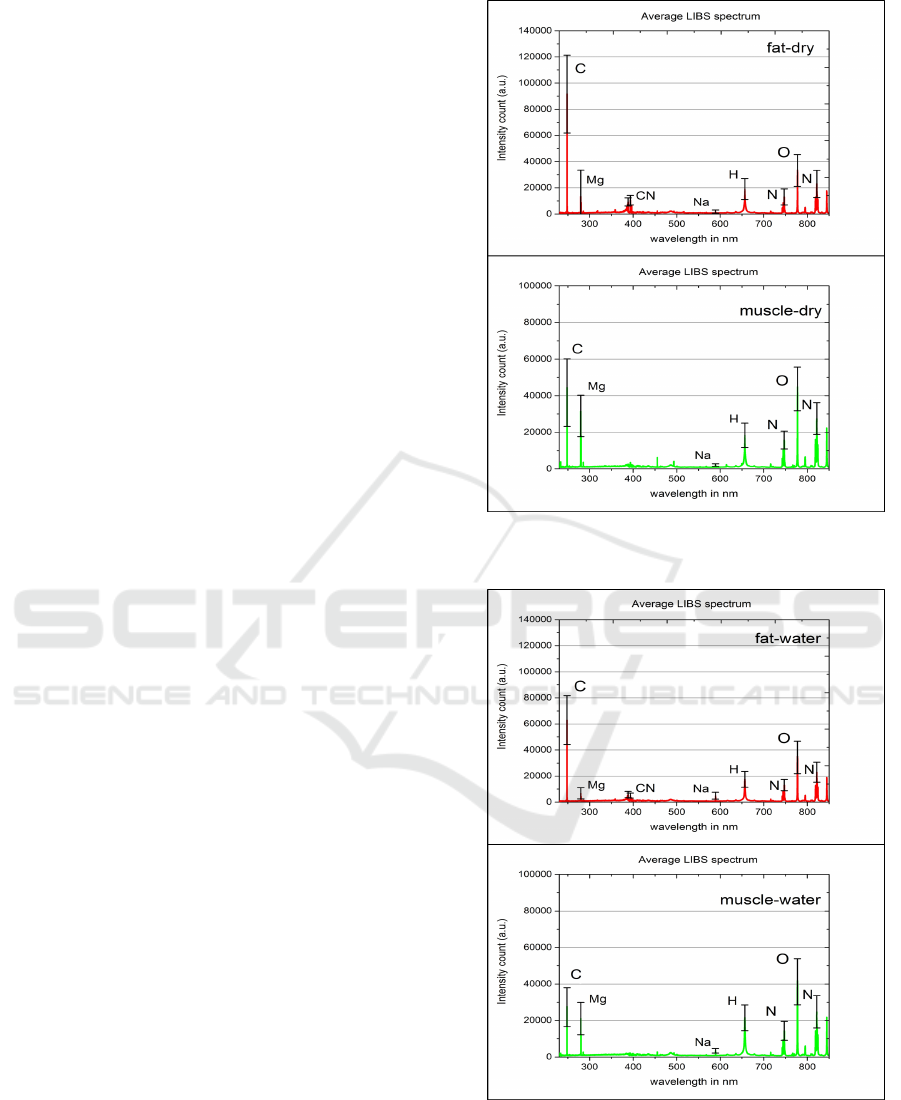
Figure 2 shows the average LIBS spectrum for dry
fat and muscle tissue, which are compared. Due to
the fact that fat tissue contains a very high amount of
carbon (Woodard and White, 1986), the maximum in-
tensity peak in the LIBS spectrum can be observed at
247.85 nm which corresponds to the emission line of
carbon species (Kramida and Ralchenko, ). Further
we observe a smaller peak at 279.55 nm that corres-
ponds to the emission line of magnesium (Mg) which
is contained in fat tissue in a small amount (Wood-
ard and White, 1986). Comparing these values to the
average spectrum of muscle tissue, the carbon peak
obtained from muscle is significantly smaller which
is expected due to the relatively much lower carbon
amount in this tissue type. The magnesium peak is
stronger in the muscle spectrum. On the other hand,
the LIBS spectrum of fat shows several significant
peaks in the spectral region 385 − 390nm which are
observed to a much lower extent for muscle tissue.
This peak region corresponds to carbon-related mo-
lecular emissions from CN (with band head at ap-
proximately 388.42 nm) which is related to the higher
carbon content of fat tissue. Other peaks of interest
for successful tissue differentiation are the sodium
peak (Na) at 589 nm, the hydrogen peak at 656.3 nm
and the oxygen peak (O) at 777.4 nm. While the so-
dium and the hydrogen peaks are very comparable for
muscle and fat tissue regarding the intensity, the oxy-
gen peak is much stronger observed for muscle tissue.
Next step is to compare the spectra of the tissues with
saline layer (Figure 3).
The most prominent change in the spectrum is
easily observed in the carbon peak at 247.85 nm for
muscle and fat tissue. Due to the liquid on the tissue
surface, the LIBS laser does not ablate as much tis-
sue material as without the liquid, because the laser
energy is now absorbed by the tissue as well as the li-
quid leading to a smaller amount of energy deposited
into the tissue material. Hence, there are less carbon
species ionized in the plasma plume. In the same way,
a decrease in the intensity values of the magnesium
and the CN peak can be observed. At the same time,
Figure 2: Measured single-pulse LIBS spectra for dry fat
and muscle tissue.
Figure 3: Measured single-pulse LIBS spectra for contam-
inated fat and muscle tissue.
the sodium peak at 589 nm increases, which can be
explained reliably by the use of saline solution that
contains 0.9 % of sodium chloride. The increased so-
LIBS based Tissue Differentiation for Er:YAG Surgical Laser
249
dium peak proves the assumption that the elements
contained in the saline solution will have an impact
on the obtained LIBS spectra of the tissue.
4.2 Double-pulse LIBS using Er:YAG
Laser
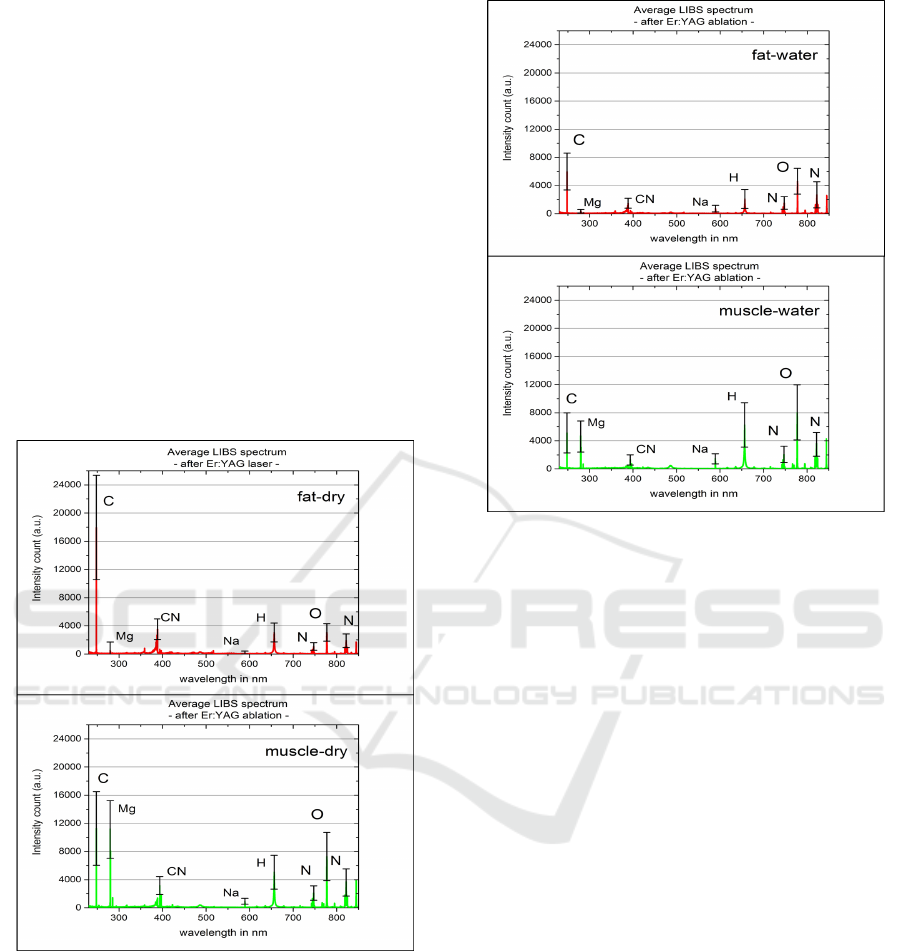
Analyzing the average LIBS spectrum of dry (Fig-
ure 4) and wet fat tissue (Figure 5) first, it can be ob-
served that the C and CN peaks significantly change.
This is due to the saline solution layer on the tis-
sue surface preventing carbonization of the underly-
ing tissue, so that less carbon species were ablated and
detected in the plasma plume. In addition to that, the
sodium (Na) peak at 589 nm increased slightly due to
the sodium content of the saline solution, which oc-
cured to a similar extent in the spectra of the single-
pulse setup.
Figure 4: Measured double-pulse LIBS spectra for dry fat
and muscle tissue.
Similar to the observations made for fat tissue,
the C and CN peaks significantly decreased in com-
parison to the average spectrum of dry muscle tissue
taken in this setup. This can be explained again by
the saline solution layer on the tissue surface lessen-
ing the process of carbonization by the Er:YAG laser.
Moreover, an increase of the sodium, hydrogen and
oxygen peaks is observed (Figure 5). Although this
may indicate that the Er:YAG laser did not ablate all
of the saline solution prior to LIBS analysis, there is
Figure 5: Measured double-pulse LIBS spectra for contam-
inated fat and muscle tissue.
another scenario potentially contributing to this be-
havior. Since the Er:YAG laser pulse is incident
shortly before the LIBS laser, ablated material by
the first laser pulse is ejected perpendicularly to the
surface and could interfere with the following LIBS
laser. The Er:YAG laser could ablate the saline solu-
tion, whose ablated particles are then ionized by the
Nd:YAG laser and therefore detected in the plasma
plume. This would explain that the Er:YAG laser in-
deed is able to clean the tissue surface, however, the
ablated material is also detected.
4.3 Classification Results
Here, we will only focus on the differentiation of the
same tissue type under the two conditions. This is be-
cause the differentiation between the two tissue types
under the two conditions should not be difficult as
their elemental composition is different and hence the
effect of the saline layer is not expected to interfere
to a level that diminishes their difference. This as-
sumption is in agreement with the results in table 1
which gives an overview on the classification results
for single-pulse and double-pulse LIBS. It shows the
sensitivity, specifity and AUC (area under curve for
ROC analysis). Classification performance is very
good for single-pulse LIBS which is considered as
disadvantageous since the same tissue type should
PHOTOPTICS 2018 - 6th International Conference on Photonics, Optics and Laser Technology
250
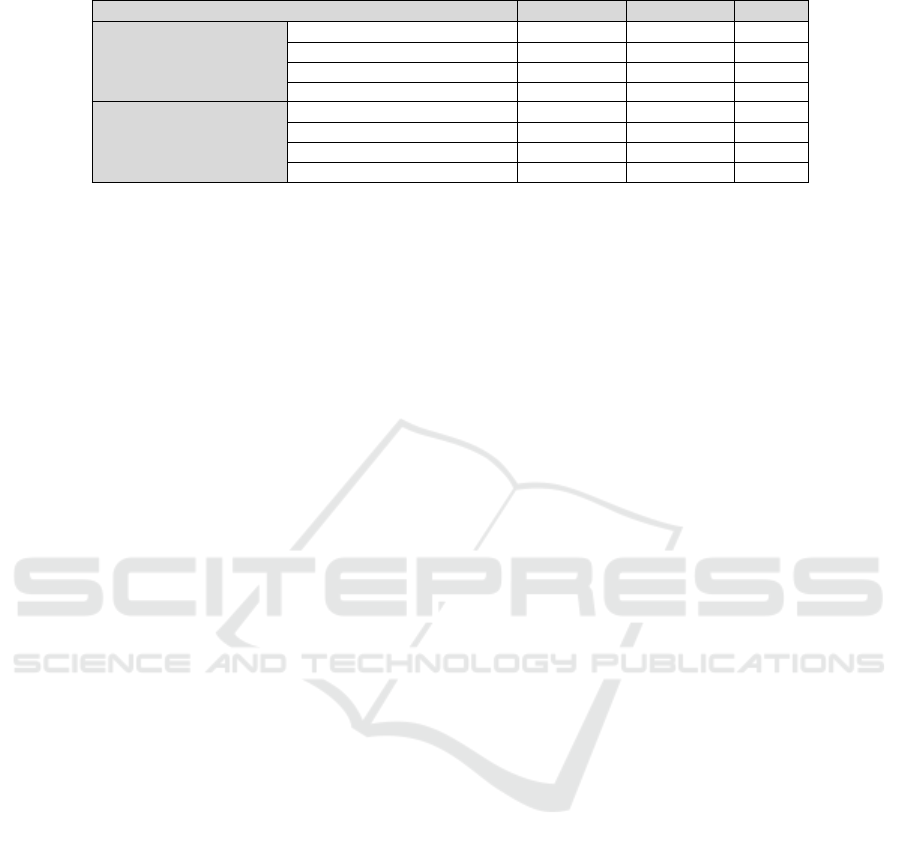
Table 1: Classification results for single -and double-pulse LIBS. The sensitivity, specificity and AUC values are shown.
Sample Combination Sensitivity Specificity AUC
Single-pulse Fat-dry / Fat-water 0.9967 0.9933 0.9987
LIBS Muscle-dry / Muscle-water 0.9133 0.9400 0.9792
Fat-dry / Muscle-dry 0.9967 0.9933 0.9987
Fat-water / Muscle-water 0.9967 0.9933 0.9995
Double-pulse Fat-dry / Fat-water 0.8000 0.8600 0.8629
LIBS Muscle-dry / Muscle-water 0.8267 0.7567 0.8364
Fat-dry / Muscle-dry 0.9733 0.9767 0.9911
Fat-water / Muscle-water 0.9300 0.9267 0.9613
not be differentiated. Comparing the average val-
ues of sensitivity, specificity and AUC for both LIBS
setups used, it becomes obvious that the classifica-
tion performance decreases for each classifier when
the Er:YAG laser is used. The cleaning effect of the
Er:YAG laser causes the average AUC level to drop by
around 13 % differentiation for fat and muscle tissue.
5 SUMMARY
The experimental results obtained in this study are ex-
pected to lay the groundwork for future soft tissue dif-
ferentiation under in-vivo conditions after the LIBS
technique has proven in recent studies to have the po-
tential for successful ex-vivo tissue differentiation.
The results in this study have shown promising pro-
spects for future in-vivo tissue differentiation using
LIBS. Considering that different kinds of body flu-
ids or liquids can be present on tissue surfaces during
surgery, it was shown that a thin saline solution layer
applied onto the surface has a significant influence on
LIBS spectra of fat and muscle tissue. Furthermore,
the study indicates that an ER:YAG laser pulse prior
to the LIBS laser pulse can serve as an in-vivo surface
cleaner leading to improved tissue classification.
In future, the influence of other possible body fluids
such as blood needs to be addressed to fully under-
stand and evaluate the potential of LIBS to perform
in-vivo tissue differentiation in the future. In addi-
tion to that, the potential influence of the liquid layer
thickness has to be investigated and other types of tis-
sue such as nerve and skin tissue have to be taken into
consideration for those investigations.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the funding of
the Erlangen Graduate School in Advanced Optical
Technologies (SAOT) by the Deutsche Forschungs-
gemeinschaft (German Research Foundation-DFG)
within the framework of the Initiative for Excellence.
REFERENCES
Cremers, D. A. and Radziemski., L. J. (2013). Hand-
book of laser-induced breakdown spectroscopy.
Wiley.
Goldberg., D. (2013). Laser Dermatology. Springer.
Kramida, A. and Ralchenko, Y. NIST Atomic Spectra
Database (version 5.4),.
Mehari, F. (2014). Laser induced breakdown spectro-
scopy for bone and cartilage differentiation - ex
vivo study as a prospect for a laser surgery feed-
back mechanism. Biomedical optics express,,
5(11).
Solomon, K. D. (2009). Lasik world literature review:
quality of life and patient satisfaction. Ophthal-
mology, 116(4).
Walter, M. (1999). Photoablation of bone by excimer
laser radiation. Lasers in Surgery and Medicine,,
25.
Woodard, H. Q. and White, D. R. (1986). The com-
position of body tissues. The British journal of
radiology,, 59(708).
LIBS based Tissue Differentiation for Er:YAG Surgical Laser
251
