
Test Device for Blood Transfusion Safety
How Acoustics Can Help Preventing Any Red Cells Incompatibility
Karine Charrière
1
, Jean-François Manceau
2
, Pascal Morel
3
, Véronique Bourcier
4
, Wilfrid Boireau
2
,
Lionel Pazart
1
and Bruno Wacogne
1,2
1
INSERM CIC 1431, Besançon University Hospital, 25000 Besançon, France
2
FEMTO-ST Institute, Univ. Bourgogne Franche-Comté, CNRS, 25030 Besançon cedex, France
3
Etablissement Français du Sang Bourgogne/Franche-Comté, 25000 Besançon, France
4
Hemovigilance Service, Besançon University Hospital, 25000 Besançon, France
Keywords: Red Cells Incompatibility, Blood Transfusion, Microsystem, Medical Device, Transfusion Safety, Acoustic
Mixing, Acoustic Detection.
Abstract: During red cells concentrates transfusion, red cells incompatibilities still occur despite the laboratory
controls based on immuno-hematologic techniques. Red cells incompatibilities appear when patient’s
antibodies bind to red cells to be transfused. Up to now, all pre-transfusion testing are addressed using
techniques based on immunology. This is time consuming, expensive and some incompatibility situations
cannot be addressed at the patient’s bedside. In this position paper, we propose a completely novel
paradigm. Our hypothesis is that red blood cells sensitized by the patient’s antibodies see their deformability
greatly reduced. This induces changes of the rheological properties of the “red cells concentrate /patient’s
blood” mixture. Studies described in this position paper aim at characterizing these modifications by
measuring the characteristics of acoustic waves propagating in the mixture and to produce a mobile and
automated acousto-micro-fluidic device which would allow detecting any incompatibility at the patient’s
bed side.
1 INTRODUCTION
In most countries, a crossmatch (a compatibility test
between blood for transfusion and the receiver's
blood) is carried out in a laboratory prior to
transfusion, but is of no use when an error occurs
after the blood has been transfused. The current
techniques for carrying out a crossmatch are either
manual, with blood reagents and samples being
mixed in tubes or being placed on gel columns
before centrifuging (e.g. Across Gel® Cross Match,
from Dia Pro or ID-Card 50531 from Bio-Rad), or
automated (e.g. the Qwalys analysers from Diagast).
As far as these automated systems are concerned, the
analysers may only be used in the laboratory and are
difficult to adapt for use at the patient's bedside, in
particular because of the need to treat the blood
samples before they are analysed.
Many countries are thus looking at solutions at
the patient's bedside in order to reduce the rate of
transfusional accidents related to avoidable
immunological incompatibilities. Currently, the final
pre-transfusional test at the patient's bedside consists
exclusively of an identity check (identity of the
blood pouch and identity of the patient). This
method cannot guarantee that there will be no
transfusional accident because 50% of the reported
adverse effects are due to human error (SHOT,
2011). Therefore, in spite of increasingly effective
safety systems, it is currently impossible to eliminate
entirely the risks due to human error, both in the
laboratory and at the time of the transfusion.
A few countries, including France, carry out a
second ABO compatibility test at the patient's
bedside immediately before the blood transfusion,
using control charts requiring several handling
procedures. These tests makes allows us to limit
fatal transfusional accidents in France, but does not
completely prevent human error, which is the main
cause of transfusional error (Linden, 2000 - Myhre,
206
Charrière, K., Manceau, J-F., Morel, P., Bourcier, V., Boireau, W., Pazart, L. and Wacogne, B.
Test Device for Blood Transfusion Safety - How Acoustics Can Help Preventing Any Red Cells Incompatibility.
DOI: 10.5220/0006635702060211
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 1: BIODEVICES, pages 206-211
ISBN: 978-989-758-277-6
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reser ved
2000 - Sazama, 2003). These errors can occur during
the test procedure at the patient's bedside, in
particular when the hemagglutination reaction is
read and interpreted (Henneman, 2007 - Myhre,
2000).
In this position paper, we propose a conceptual
and technical innovative approach which will
address any red cells incompatibility in a mobile and
easy to use medical device.
The scientific hypotheses can be summarized as
follows. Red cells are nucleus-free cells with an
average diameter of 7.2 µm. Their cytoplasm is rich
in haemoglobin. Their membrane is extremely
flexible. This makes them deformable enough to
propagate in the much smaller blood capillary
network (Kim, 2015 – Tomaiuolo, 2014 –
Mohandas, 2008). Physiological aging or various
pathological situations jeopardize this deformability
(Franco, 2013 – Mourao, 2016 – Chien, 1987).
Among the factors, deformability is reduced when
red cells are covered in antibodies. More
importantly, a loss in red cell deformability results in
a significant increase of blood viscosity (Kim,
2015).
When the red cells concentrate is compatible
with patient’s blood, antigens at the surface of the
red cells to be transfused are not complementary to
antibodies present in the patient’s blood. No
antigen/antibody reaction occurs and deformability
of the red cells to be transfused is not modified.
Transfusion is then allowed.
On the contrary, when the red cells concentrate is
incompatible, antigens at the surface of the red cells
to be transfused are recognized by the patient’s
antibodies. This induces an immune reaction whose
consequences are more or less critical
(alloimmunization, haemolytic reaction, patient’s
death). Our hypothesis is that fixation of antibodies
on the red cells to be transfused reduces their
deformability and alters the rheological properties of
the “red cells concentrate (blood bag)/patient’s
blood” mixture. This alteration can be measured
using acousto-fluidic interactions. In this case, no
supplementary reagent is required and any immune
incompatibility situation can be detected.
This communication is organized as follows. In
section 2, we present a schematic representation of
the foreseen medical device. In part 3, we present
preliminary results obtained concerning acoustic
mixing, activation and detection of liquid
rheological modifications. In part 4, and in line with
the scope of a position paper, we present scientific
and socio-economic impacts such a device could
address.
2 DESCRIPTION OF THE
FORESEEN DEVICE
The device under development includes studies on
microfluidics, acoustic mixing, acoustic activation
and acoustic detection. Examples of acoustic
manipulation of liquids will be presented in section
3. In this section, we detail the biological and
technological studies currently under investigation.
At the cellular level, deformability of red cells is
investigated using conventional techniques: Atomic
Force Microscopy, possibly optical tweezers and
quantitative phase imaging (Kim, 2015).
Experiments are conducted in condition as close as
possible to real clinical conditions. For example, we
are setting up experiments with compatibility or
incompatibility situations induced by natural and/or
unexpected antibodies against red cell antigens.
Variations of the rheological properties of the bag’s
cell/patient’s blood mixture will be investigated
using a device such as the one described in figure 1
in the case of an incompatibility.
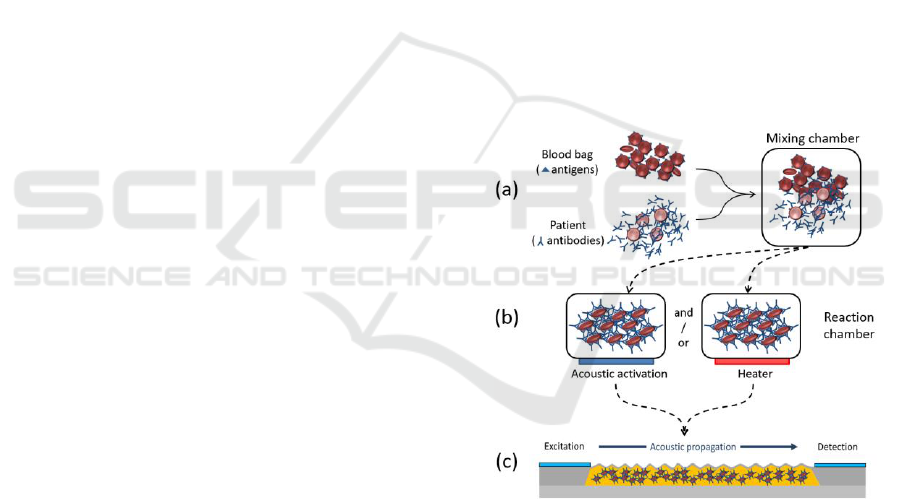
Figure 1: Schematic representation of the foreseen device.
(a) Driving the samples to the mixing chamber. (b)
Reaction activation either by acoustic activation or
heating. (c) Acoustic detection of incompatibilities.
Patient’s blood and bag’s red cell will be
transferred to a mixing chamber (part (a) in figure
1). The goal is to make the mixture as homogeneous
as possible in order to optimize recognition of the
red cell antigens by antibodies from the patient.
Mixing by diffusion would be too long for a
practical use at the patient’s bed side. Therefore,
acoustic mixing comparable to what is presented in
section 3 will be employed.
Test Device for Blood Transfusion Safety - How Acoustics Can Help Preventing Any Red Cells Incompatibility
207

The blood mixture will be transferred in a
reaction chamber as illustrated in part (b) of the
figure. In order to reduce the time required for the
antigen/antibody reaction, this chamber may have to
be temperature controlled and/or equipped with an
acoustic activation device as presented in the next
section.
Once the antigen/antibody reaction is completed,
the mixture is then transferred into a test chamber
equipped with acoustic transducers (part (c) in the
figure). The transducer generates acoustic waves
(Lamb waves in this case) which propagate through
the mixture and are detected by the acoustic
detector. This detector will detect variations of the
rheological properties of the mixture through
measurement of both amplitude and phase by means
of an integrated network analyzer. In this case,
measurement obtained with the acoustic detector
will have to be compared to values obtained in the
case of compatibility. This may become an issue
since referenced or calibrated measurement are
difficult to perform in an automated way at the
patient’s bed side. Also, Lamb waves only interact
with liquid in the vicinity of the membrane. A more
bulky architecture may be required in order to
enhance the sensor’s sensitivity.
A possible simpler architecture is describe in
figure 2. Here only one chamber and only one
transducer is used. Red cells to be transfused and
patient’s blood are injected in the chamber and the
piezoelectric transducer is driven with frequencies
and amplitudes suitable for either fluid mixing,
activation or reaction sensing. The acoustic
transducer may be patterned so that several acoustic
modes can be generated. Natures of the most
suitable acoustic modes will have to be defined for
the aimed function. Next, the same transducer is
used to monitor the time-dependent evolution of the
acousto-fluidic properties of the mixture during the
antigen/antibody reaction. This make the method
potentially reference or calibration free.
We already mentioned that the age of red cells
influences their membrane deformability (Franco,
2013). If needed, micro-filtration units will be added
to the device in order to remove aged red cells.
These units will be inserted in the circuitry
employed to transfer the red cells to be transfused
and the patient’s blood to the mixing chamber (part
(a) in figure 1). However, a device similar to the one
we propose for blood incompatibility could be used
in order to qualify the age of red cells contained in
transfusion bags. This constitutes one of the
scientific impacts we describe in section 4.
Figure 2: Simplified architecture with one sensor/actuator.
3 ACOUSTIC INTERACTIONS
WITH FLUIDS
In section 3, we mentioned that acoustic
manipulation is used for fluid mixing,
antigen/antibody reaction activation and rheological
properties monitoring. In what follows, we present
preliminary results concerning acoustic fluids
manipulations which can be directly adapted for
blood compatibility assessment.
3.1 Acoustic Mixing
We demonstrated acoustic mixing using patterned
acoustic transducers (Kardous, 2014). This structure
allowed exciting different acoustic modes in a liquid
droplet as illustrated in figure 3.
Figure 3: (a): Generation of acoustic mode (5.5). (b)
Generation of a degenerated mode (1,3)+(3,1). Figure
adapted from (Kardous, 2014).
Mixing various viscous fluids have been tested.
Liquids to be mixed are rhodamine in water and
40% glycerol in water. Fluorescence of rhodamine is
used to visualize the mixing of these two liquids.
The result is shown in figure 4. In this figure,
negative times indicate that no acoustic activation is
applied. For positive times, acoustic mixing is
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
208
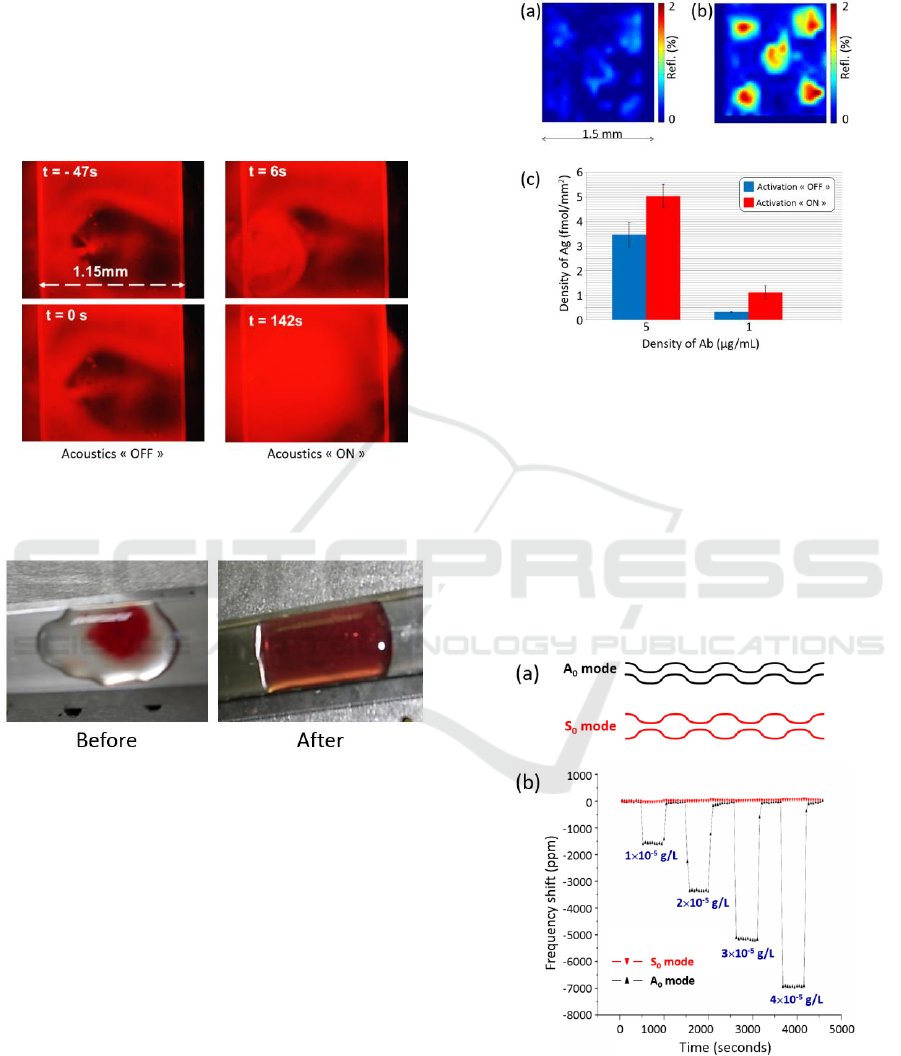
applied. It can be noted that the two liquid phases
are completely mixed after about 3-5 min.
Acoustic mixing was also demonstrated in order
to homogenize blood red cells in a physiological
serum droplet. This is illustrated in figure 5. Note
that acoustic manipulation can also be used for
reversible concentration/dispersion of micro-
particles in liquids. This is not shown here because it
is out the scope of this communication.
Figure 4: Acoustic mixing of water and glycerol solution.
Rhodamine is used to visualize mixing using fluorescence.
Figure adapted from (Kardous, 2014).
Figure 5: Acoustic homogenization of red cells in a
physiological serum droplet.
3.2 Acoustic Activation
Antigen/antibody recognition can be enhanced using
acoustic activation during antibodies immobilization
step (Kardous, 2011). Monoclonal antibodies
A9H12 were deposited in droplets of 400 nL at the
surface of membrane compatible with Surface
Plasmon Resonance imaging (SPRi) experiments.
Five antibodies spots were deposited. The
corresponding antigen was LAG-3 proteins. Figure 6
shows SPR images of the biochip were (a)
corresponds to the capture of LAG-3 without
acoustic activation and (b) corresponds to the same
spots when acoustic activation is used.
Amplification of the bio-recognition ranges from 1.5
to 3. This is illustrated in figure 6(c) and it highlights
the efficiency of acoustic mixing regarding
antigen/antibody reaction.
Figure 6: Acoustic activation of antigen/antibody
recognition. (a) LAG-3 antigens captured by A9H12
antibodies on a SPRi biochip without acoustic activation.
(b) The same experiment using acoustic activation. (c)
Amplification rate obtained for 2 values of the antibodies
concentrations. Figure adapted from (Kardous, 2010).
3.3 Acoustic Sensing
Acoustic sensing of a fluid may be achieved using
the Lamb waves. Various Lamb waves exist such as
symmetric modes noted S
i
and the anti-symmetric
modes noted A
i
. A
0
and S
0
are shown in figure 7(a).
Figure 7: Acoustic sensing. (a) Anti-symmetric and
symmetric Lamb waves modes. (b) Sensing solutions of
varying concentrations in sodium chloride.
Each mode exhibits its own resonant frequency
and penetration depth in the liquid under
Test Device for Blood Transfusion Safety - How Acoustics Can Help Preventing Any Red Cells Incompatibility
209

investigation. An example of acoustic sensing is
given in figure 7(b). Here, resonant frequencies of
the modes are about a few MHz. Lamb waves were
used to monitor the concentration in sodium
chloride. Addition of sodium chloride increases the
density of water. This results in shifts of the resonant
frequency of the acoustic mode. The figure clearly
shows that acoustic sensing allows measuring the
sodium chloride concentration (i.e. the viscosity)
when A
0
mode is excited. S
0
mode does not
experience frequency shifts due to its inadequate
penetration depth.
4 SCIENTIFIC AND SOCIO-
ECONOMICAL IMPACTS
As mentioned above and in the scope of a position
paper, we present the scientific and socio-economic
impacts such a device potentially produce in the next
section.
The new paradigm which constitutes the acoustic
detection of immuno-incompatibilities potentially
allows detecting any cause of incompatibility. Given
the increasing exchanges of populations and the
increasing number of multiple transfusions, some
unexpected antibodies against red cell antigens are
not currently detected or identified. The acoustic
technique we propose here would detect
incompatibility without the need of prior
identification.
Outside the field of transfusion safety, studies are
ongoing concerning the effect of the age of the red
cells concentrates on the transfusion efficiency
(Vallion, 2015 – Lacroix, 2015 – Lapierre, 2007 –
Desmarets, 2016). In 2008, Luten et al. showed that
about 30% of bag’s red cell are eliminated by the
organism within 24 hours after transfusion, probably
because of the loss in membrane deformability
(Luten, 2008). Therefore, a device like the one we
propose could be adapted in order to perform a
selection of the red cells based on the detection of
the less deformable cells at the moment of blood
donation. This potentially would improve blood
transfusion efficiency.
By changing the principle of incompatibility
detection, this acousto-fluidic device will be a
technological advance because no expensive
antibodies will be used (human IgGs). The foreseen
cost of the disposable part of the device is estimated
to about 5$.
Technology development related to this device
will considerably simplify ultimate compatibility
controls by proposing a mobile device which can be
used by non-trained staff at the patient’s bed side. In
most countries, organization of care will benefit
from this technological and conceptual
breakthrough. In countries where the transfusion
chain (from donation to transfusion) is well
organized, this device will contribute to the
harmonization of pre-transfusion controls as
advocated by the World Health Organization. In
countries where the transfusion chain is not or
partially organized, such a device will offer a cost-
effective solution to enhance blood transfusion
safety. In the future, this device could be proposed
for every red cell concentrates transfusion which
means at least 17 million tests in Europe, 20 million
in the US and 140 million worldwide.
In the world, only 62% of countries have a
legislation and care organization concerning the
quality and the security of blood transfusions. In
2016, 40 countries admit that no qualification of the
blood donation is performed due to the lack in
qualified staff and economic issues (WHO
factsheets).
The World Health Organization highlights the
need for international standardization of safety
processes, in particular for what concerns blood
incompatibilities. Simplifying the blood transfusion
safety controls would improve the access to safe and
cost-effective blood transfusions in more countries
than it is today and to the benefit of a rapidly
increasing population.
5 CONCLUSIONS
In this position paper, we have described how
immuno-erythrocytic incompatibilities can have
severe or lethal consequences. We pointed out that
there exists no method to address all the
incompatibility situations.
Here, we propose a new paradigm based on the
use of acoustic sensing to detect incompatibilities.
The hypothesis is that when red cells from the red
cells concentrate are covered in antibodies issued
from the patient’s blood, the deformability of their
membrane is strongly reduced. As a consequence,
rheological properties of the mixture red cell
concentrate/patient’s blood are modified and these
modifications can be acoustically detected.
Furthermore, this acoustic detection is independent
of the immunologic origin of a possible
incompatibility. Examples of already demonstrated
acoustic mixing, activation and sensing using other
fluids have been presented. The preliminary results
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
210

demonstrate the feasibility of the method we
propose. Experimental studies are currently ongoing
using whole blood samples issued from donators and
red cell concentrates.
When completed, these studies will lead to a
global solution able to address any incompatibility
situation which can be used by non-specialized staff
directly at the patient’s bed side. This
incompatibility detection has to be coupled to an
identification of the antibody / antigen responsible of
the immunological reaction. This technology will
reinforce transfusion safety in countries where the
transfusion chain is already organized. At the same
time, such a device will offer an affordable solution
to enhance blood transfusion safety in other
countries.
ACKNOWLEDGEMENTS
This work is partially funded by the “translational
PEPS call” from the CNRS. Operation 2017.
REFERENCES
Chien, S. 1987, Red Cell Deformability and its Relevance
to Blood Flow. Annu. Rev. Physiol. Vol. 49, pp. 177–
192.
Desmarets, M., Bardiaux, L., Benzenine, E., Dussaucy, A.,
Binda, D., Tiberghien, P., Quantin, C. and Monnet, E.,
2016, Effect of storage time and donor sex of
transfused red blood cells on 1-year survival in
patients undergoing cardiac surgery: an observational
study, Transfusion, Vol. 56, pp. 1213–1222.
Franco, R.S., Puchulu-Campanella, M.E., Barber, L.A.,
Palascak, M.B., Joiner, C.H., Low, P.S. and Cohen,
R.M., 2013, Changes in the properties of normal
human red blood cells during in vivo aging. Am. J.
Hematol., Vol. 88, pp. 44–51.
Henneman, E.A., Avrunin, G.S., Clarke, L.A., Osterweil,
L.J., Andrzejewski, C., Jr, Merrigan, K., Cobleigh, R.,
Frederick, K., Katz-Bassett, E., Henneman, P.L., 2007.
Increasing patient safety and efficiency in transfusion
therapy using formal process definitions. Transfus.
Med. Rev. 21, 49–57. doi:10.1016/j.tmrv.2006.08.007.
Kardous, F., Rouleau, A., Simon, B., Yahiaoui, R.,
Manceau, J.F. and Boireau, W., 2010, Improving
immunosensor performances using an acoustic mixer
on droplet microarray, Biosens. Bioelec., Vol. 26, pp.
1666-1671.
Kardous, F., Yahiaoui, R., Aoubiza, B. and Manceau, J.F.,
2014, Acoustic mixer using low frequency vibration
for biological and chemical applications, Sens. Act. A,
Vol. 211, pp. 19-26.
Kim, J., Lee, H. and Shin, S., 2015, Advances in the
measurement of red blood cell deformability: A brief
review. J. Cell. Biotechnol., Vol. 1, pp. 63–79.
Lacroix, J. et al., 2015, Age of transfused blood in
critically ill adults. N. Engl. J. Med., Vol. 372, pp.
1410–1418.
Lapierre, V., Aupérin, A., Robinet, E., Ferrand, C.,
Oubouzar, N., Tramalloni, D., Saas, P., Debaene, B.,
Lasser, P. and Tiberghien, P., 2007, Immune
modulation and microchimerism after unmodified
versus leukoreduced allogeneic red blood cell
transfusion in cancer patients: results of a randomized
study - Transfusion - Wiley Online Library. Available
at: http://onlinelibrary.wiley.com/doi/10.1111/j.1537-
2995.2007.01344.x/full.
Linden, J.V., Wagner, K., Voytovich, A.E., Sheehan, J.,
2000. Transfusion errors in New York State: an
analysis of 10 years’ experience. Transfusion (Paris)
40, 1207–1213.
Luten, M., Roerdinkholder-Stoelwinder, B., Schaap, N.P.,
de Grip, W.J., Bos, H.J. and Bosman GJ., 2008,
Survival of red blood cells after transfusion: a
comparison between red cells concentrates of different
storage periods, Transfusion, Vol. 48, pp. 1478–1485.
Mohandas, N. and Gallagher, P. G., 2008, Red cell
membrane: past, present, and future, Blood, Vol. 112,
pp. 3939–3948.
Mourão, L.C., Roma, P.M., Sultane Aboobacar Jda, S.,
Medeiros, C.M., de Almeida, Z.B., Fontes, C.J.,
Agero, U., de Mesquita, O.N., Bemquerer, M.P. and
Braga, É.M., 2016, Anti-erythrocyte antibodies may
contribute to anaemia in Plasmodium vivax malaria by
decreasing red blood cell deformability and increasing
erythrophagocytosis, Malar. J. Vol. 15, pp. 397.
Myhre, B.A., McRuer, D., 2000. Human error-a
significant cause of transfusion mortality. Transfusion
(Paris) 40, 879–885.
Sazama, K., 2003. Transfusion errors: scope of the
problem, consequences, and solutions. Curr. Hematol.
Rep. 2, 518–521.
SHOT, 2011. Annual SHOT report 2011.
South, S.F., Casina, T.S., Li, L., 2012. Exponential error
reduction in pretransfusion testing with automation.
Transfusion (Paris) 52, 81S–87S. doi:10.1111/j.1537-
2995.2012.03816.x.
Tomaiuolo, G. 2014, Biomechanical properties of red
blood cells in health and disease towards
microfluidics, Biomicrofluidics, Vol. 8, pp. 51501.
Vallion, R., Bonnefoy, F., Daoui, A., Vieille, L.,
Tiberghien, P., Saas, P. and Perruche, S., 2015,
Transforming growth factor-β released by apoptotic
white blood cells during red blood cell storage
promotes transfusion-induced alloimmunomodulation,
Transfusion, Vol. 55, pp. 1721–1735.
World Health Organization, Blood safety and availability.
Available at: http://www.who.int/mediacentre/
factsheets/fs279/en/.
Test Device for Blood Transfusion Safety - How Acoustics Can Help Preventing Any Red Cells Incompatibility
211
