
A Rule-based Method Applied to the Imbalanced Classification of
Radiation Toxicity
Juan L. Domínguez-Olmedo
1
, Jacinto Mata
1
, Victoria Pachón
1
and Jose L. Lopez Guerra
2
1
Escuela Técnica Superior de Ingeniería, University of Huelva, Huelva, Spain
2
Department of Radiation Oncology, University Hospital Virgen del Rocío, Sevilla, Spain
Keywords: Imbalanced Data Classification, Rules Discovery, Prostate Cancer.
Abstract: This paper describes a rule-based classifier (DEQAR-C), which is set up by the combination of selected
rules after a two-phase process. In the first phase, the rules are generated and sorted for each class, and then
a selection is performed to obtain a final list of rules. A real imbalanced dataset regarding the toxicity during
and after radiation therapy for prostate cancer has been employed in a comparison with other predictive
methods (rule-based, artificial neural networks, trees, Bayesian and logistic regression). DEQAR-C
produced excellent results in an evaluation regarding several performance measures (accuracy, Matthews
correlation coefficient, sensitivity, specificity, precision, recall and F-measure) and by using cross-
validation. Therefore, it was employed to obtain a predictive model using the full data. The resultant model
is easily interpretable, combining three rules with two variables, and suggesting conditions that are mostly
confirmed by the medical literature.
1 INTRODUCTION
Prostate cancer (PC) is the most commonly
diagnosed cancer affecting men, and the third
leading cause of death in men in Europe (Ferlay et
al., 2013). The American Cancer Society estimated
that more than 200,000 men are diagnosed in the
United States with 30,000 deaths (American Cancer
Society, 2014). Although there is an improvement in
tumor control rates using radiation dose escalation,
PC radiotherapy is limited by the proximity of
surrounding normal tissues and because of the
observed dose-effect association with toxicity. It is
essential to understand the true complications
associated with doses delivered to normal anatomy,
to ensure the delivery of a sufficient dose with
minimal complications. The use of intensity-
modulated and image-guided radiation therapy can
decrease acute toxicity in PC patients (Valeriani et
al., 2013); (Morimoto et al., 2014).
Within the field of artificial intelligence and,
more specifically in machine learning, one of the
methods employed to extract knowledge from data is
the use of association rules. Association rule mining
is a technique whose purpose is to extract strong and
interesting relationships between patterns in a set of
data. An association rule takes the form A → C,
where A (the antecedent) and C (the consequent)
express a condition (or a conjunction of conditions)
on variables of the dataset (Agrawal et al., 1993);
(Rudin et al., 2013). The measures support and
confidence are used to assess the quality and
importance of the association rules. The support
measure evaluates the number of cases in which
both the antecedent and the consequent of the rule
hold. The confidence measure is the ratio between
the support of the rule and the number of cases in
which the antecedent holds. In order to filter the
usual huge number of rules generated, the values
minsup (minimum support) and minconf (minimum
confidence) are the thresholds that a rule has to
satisfy to be considered of interest.
Subgroup discovery is a type of descriptive
induction whose objective is to generate models
based on rules using a predictive perspective. It
emerged as the task of discovering properties of a
population by obtaining simple (but significant)
rules, using only one variable in the consequent: the
class or target variable (Wrobel, 1997); (Gamberger
et al., 2003); (Domínguez-Olmedo et al., 2015).
And also, numerous techniques have been
proposed for classification problems. In this kind of
Domínguez-Olmedo, J., Mata, J., Pachón, V. and Lopez-Guerra, J.
A Rule-based Method Applied to the Imbalanced Classification of Radiation Toxicity.
DOI: 10.5220/0006586401470155
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 5: HEALTHINF, pages 147-155
ISBN: 978-989-758-281-3
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
147

task, a predictive model (classifier) tries to predict,
with some certainty, the objective variable (of
categorical type). Some examples of predictive
methods are artificial neural networks, decision
trees, logistic regression and Bayesian networks,
among others (Hastie et al., 2009); (Golhar et al.,
2017); (Cortés et al., 2015); (Liu et al., 1998). In
binary classification tasks with imbalanced data, as
in the case at hand, most algorithms are not usually
capable of obtaining good results for the minority
class and, therefore, the overall classification
performance does not reach adequate values (Sun et
al., 2009); (Rastgoo et al., 2016). The technique
proposed in this paper has achieved an improved
precision in both classes, thanks in part to an
alternating selection of rules for each class.
The rest of the paper is organized as follows.
Section 2 gives a description of the methods
employed in this work. The experimental setup is
presented in Section 3. Section 4 describes the
experimental results and discussion. And the last
section presents the conclusions.
2 METHODS EMPLOYED
2.1 Description of DEQAR-C
DEQAR-C is a rule-based classifier that works by
using a list of selected rules and a default class, both
of them obtained during the training process. Figure
1 illustrates this training process, which is composed
of two phases.
In the first phase, the "rule generation phase",
DEQAR-C generates rules from the training dataset.
This generation of rules is based on a method
developed to extract knowledge in the form of
association rules (Domínguez-Olmedo et al., 2011);
(Domínguez-Olmedo et al., 2012); (Domínguez-
Olmedo and Mata, 2016). It employs a deterministic
approach to generate rules without a previous
discretization of the numerical variables. Instead of
discretizing, what may result in suboptimal results
(Grosskreutz and Ruping, 2009), the process uses a
dynamic generation of conditions. DEQAR-C
obtains an ordered list of rules (called ranking) for
each possible value of the class variable, storing
separately the best rules in each class according to
their values of confidence and support.
In the second phase, the "rule selection phase", a
selection of the rules from all the rankings is done,
by starting in the ranking of the rule with the highest
confidence-support value and alternating iteratively
between these rankings to select rules from them.
The parameter maxrules determines the maximum
number of rules that will form the classifier. Figure
2 presents an example of this rule generation for a
dataset with two classes, and also shows a possible
selection of rules using a value of 3 for maxrules.
Figure 1: Training process in DEQAR-C.
The detail of the final selection process is shown
in Algorithm 1, which takes as input the set of cases
in the training dataset, the rankings of rules and the
parameter maxrules. After starting in the ranking
with the best rule (step 3), the process continues
selecting rules from the different rankings, but only
those rules covering some case not covered by a rule
previously selected (step 8). In the case of a binary
classification, the process would alternate in the
selection of rules for the two possible classes (if
there were still rules not processed in both rankings).
The procedure stops when all the rules have been
processed or the number of selected rules reaches
maxrules (step 17). At the end, the default class will
be the one having the greatest number of cases not
covered by any of the selected rules.
HEALTHINF 2018 - 11th International Conference on Health Informatics
148
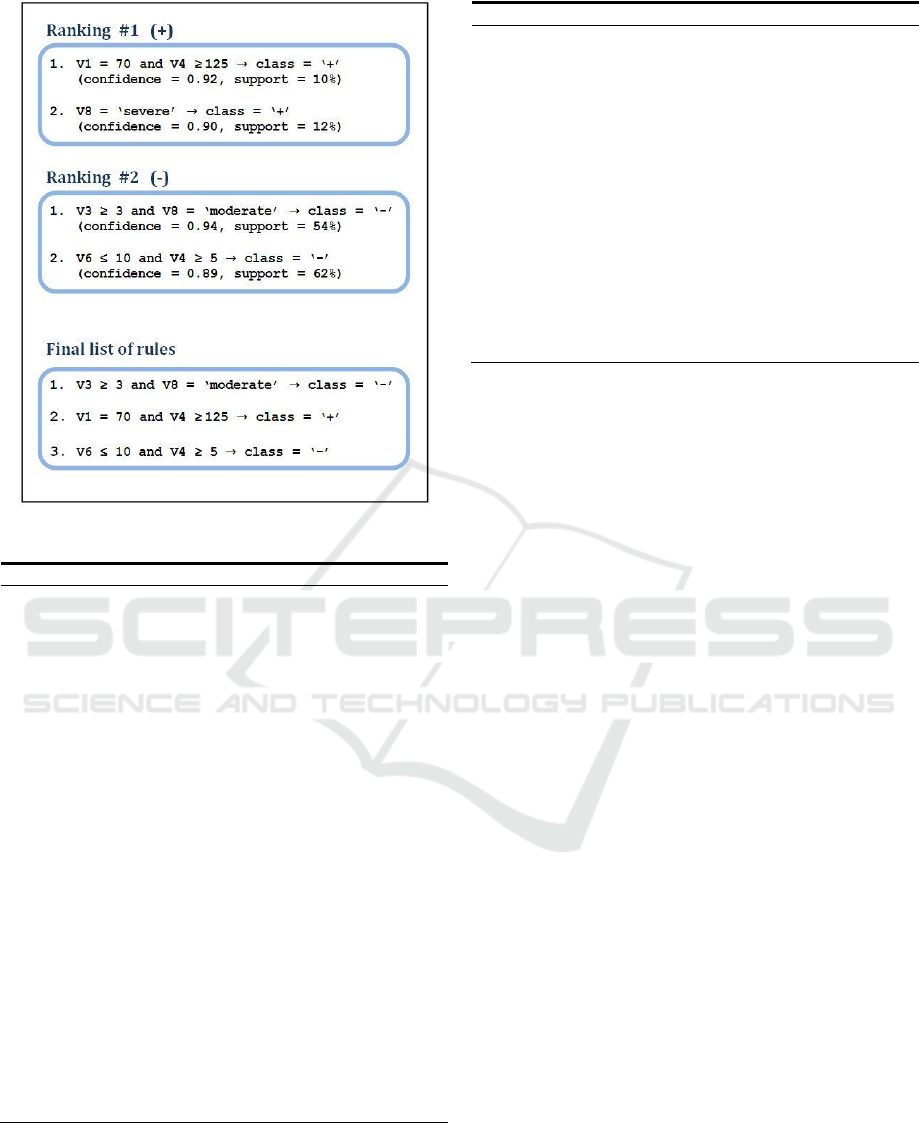
Figure 2: Example of the generation and selection of rules.
Algorithm 1: Rules Selection in DEQAR-C.
Input: training dataset, rankings of rules, maxrules
Output: final list of rules, default class
1: T = set of cases in the training dataset
2: nRules = 0
3: r = ranking with the best rule according
to the highest confidence-support value
4: stop = FALSE
5: while NOT stop do
6: if ranking r has more rules to process
then
7: R = next rule in ranking r
8: if rule R covers at least one case
in T then
9: add R to the final list of rules
10: nRules = nRules + 1
11: T = T - cases covered by rule R
12: r = next ranking (alternate)
13: end if
14: else
15: r = next ranking (alternate)
16: end if
17: if all the rules were processed OR
nRules = maxrules then
18: stop = TRUE
19: end if
20: end while
21: return the list of selected rules and
the default class
To classify a new case, DEQAR-C will search
the list of rules for the first one where the case
matches its antecedent, assigning the class of that
rule. If no rule is found, the default class is assigned.
Algorithm 2 shows this process.
Algorithm 2: Classification process in DEQAR-C.
Input: list of rules, default class, a new case to classify
Output: predicted class
1: r = 1
2: matched = FALSE
3: while NOT matched AND r ≤ number of
rules do
4: if the case matches the antecedent of
rule r then
5: predictedClass = class of rule r
6: matched = TRUE
7: end if
8: r = r + 1
9: end while
10: if NOT matched then
11: predictedClass = default class
12: end if
13: return predictedClass
2.2 Classifiers Used in the Comparison
Several predictive methods have been employed in a
comparison with DEQAR-C. Methods based on
rules (ZeroR, PART), artificial neural networks
(MultilayerPerceptron), trees (J48, RandomForest),
Bayes (BayesNet, NaiveBayes) or logistic regression
(Logistic) have been used. Some of their
characteristics are shown below:
ZeroR. It is a classification method that only
relies on the target variable (class), simply
predicting the majority class. It can be useful to
determine a baseline performance.
PART. It generates a decision list by using a
separate-and-conquer strategy (Frank and
Witten, 1998).
MultilayerPerceptron. A classifier that uses an
artificial neural network with backpropagation.
The nodes in this network are all sigmoid
(Rumelhart et al., 1986).
J48. It uses a pruned or unpruned C4.5 decision
tree (Quinlan, 1993). A decision tree builds a
classification model in the form of a tree
structure.
RandomForest. It constructs a forest of random
trees, an ensemble learning method for
classification, regression and other tasks
(Breiman, 2001).
BayesNet. It employs a Bayes network, a
probabilistic graphical model that represents a
set of random variables and their conditional
dependencies (Pearl, 1985).
NaiveBayes. It is based on Bayes theorem with
independence assumptions between predictors.
Despite its simplicity, it often outperforms more
sophisticated classification methods (John and
Langley, 1995).
A Rule-based Method Applied to the Imbalanced Classification of Radiation Toxicity
149

Table 1: Variables and units of the dataset.
Variable
Units/Values
Age
years
Indication treatment
Post-prostatectomy , Primary Prostate Cancer, Recurrence
Radiation technique
Tomotherapy, RapidArc
Gleason score
2..10
T stage
T1 , T1b , T1c , T2 , T2a , T2b , T2c , T3 , T3a , T3b , T4
Diagnosis PSA
1
ng/mL
Risk
Low, Intermediate, High
ADT
2
No ADT, Short Term, Long Term
Radiation time
days
Planning tumor volume
cc
Prostate radiation dose
Gy
Fractionation
Gy
Pelvic treatment
Yes, No
Bladder volume
cc
Bladder mean dose
Gy
Bladder median dose
Gy
GU acute toxicity
+, -
1
PSA: prostate specific antigen
2
ADT: androgen deprivation therapy
Logistic. It builds a multinomial logistic
regression model with a ridge estimator (Le-
Cessie and van Houwelingen, 1992).
3 EXPERIMENTAL SETUP
3.1 Dataset Description
In this work, a dataset about the toxicity effects
during and after treatment of PC (Lopez et al., 2015)
has been used. This dataset includes the clinical (i.e.
age), pathological (i.e. Gleason score, T score), and
therapeutic (i.e. radiation dose, fractionation, whole
pelvic lymph node irradiation, radiation technique)
information as well as the out-come (acute
genitourinary [GU] toxicity) of 162 PC patients
treated with arc radiation therapy from June 2006
through May 2012 at two institutions from different
nationalities (Europe and Latin-America).
The names of the 17 selected variables in the
dataset are shown in Table 1. The numerical
variables are 10 and the class variable is binary ('+'
for a toxicity grade ≥ 2, '-' for a toxicity grade < 2),
with a distribution for class '+' of 23.5% of the cases.
Therefore, it is an imbalanced dataset with a 3.3:1
ratio of negative/positive cases.
Ethical Considerations. All identifiable
information about the patients was adequately
removed from the da-ta to preserve anonymity.
3.2 Evaluation Criteria
In a binary classification problem, such as the one
we are presenting, we can denote with TP (True
Positive) the number of positive cases correctly
classified, with TN (True Negative) the number of
negative cases correctly classified, with FN (False
Negative) the number of positive cases incorrectly
classified, and with FP (False Positive) the number
of negative cases incorrectly classified.
The following evaluation measures were
employed in the comparison: accuracy, Matthews
correlation co-efficient, the average value of
sensitivity and specificity, precision, recall and F-
measure. A description of these measures is
presented below:
Accuracy: the proportion of true results (both
true positives and true negatives) among the
total number of cases examined.
Accuracy =
FP+FN+TN+TP
TN+TP
(1)
MCC: Matthews correlation coefficient, which
measures the quality of binary classifications
(Matthews, 1975).
MCC =
FN)+(TNFP)+(TNFN)+(TPFP)+(TP
FNFP-TN TP
(2)
HEALTHINF 2018 - 11th International Conference on Health Informatics
150

Average value of sensitivity and specificity:
sensitivity is the proportion of positives cases
that are correctly identified as such, and
specificity is the proportion of negatives cases
that are correctly identified as such.
avg (Se, Sp) =
5.0
FP+TN
TN
FN+TP
TP
(3)
Precision: analogous to positive predictive value
(PPV).
precision =
FP+TP
TP
(4)
Recall: analogous to sensitivity.
recall =
FN+TP
TP
(5)
F-measure: the harmonic mean of precision and
recall.
F-measure =
2
ecallrprecision
ecallr precision
(6)
4 EXPERIMENTAL RESULTS
4.1 Results of the Comparison
The classifiers previously mentioned were evaluated
in the task about the prediction of toxicity effects in
the radiotherapy treatment of PC. The evaluation
measures were calculated by using stratified 10-fold
cross-validation. Cross-validation reduces the
variance of the estimates and improves the
estimation of the generalization performance. In k-
fold cross-validation, the original data is partitioned
into k equal size subsets. Then, a single subset is
retained as the validation data and the remaining k-1
subsets are used as training data. The process is
repeated k times, with each of the k subsets used
exactly once as the validation data (Arlot and
Celisse, 2010). At the end, the final validation result
is calculated from all the partial results.
The machine learning software Weka (Frank et
al., 2016) was used to run the classifiers ZeroR,
PART, MultilayerPerceptron, J48, RandomForest,
BayesNet, NaiveBayes and Logistic. For a fair
comparison, the final values of the parameters used
in all the classifiers were the ones that yielded the
best results after testing several combinations of
values (grid search).
The results for accuracy, MCC and average
value of sensitivity and specificity are displayed in
Table 2 and Figure 3. As can be seen, DEQAR-C
obtained excellent results, which seems to support
the proposed selection of high confidence-support
rules for each class, not only to obtain high values of
general accuracy but also to get a satisfactory
prediction for both classes. The imbalance in the
dataset (38 positive cases and 124 negative cases)
adds more difficulty to the classification task. The
results of DEQAR-C were the best regarding these
three evaluation measures. The classifiers
NaiveBayes and MultilayerPerceptron also obtained
good results, but the difference for MCC, in
comparison with DEQAR-C, is important. Matthews
correlation coefficient is generally regarded as being
one of the best measures to describe the confusion
matrix of true and false positives and negatives by a
single number, especially suitable to the case of
imbalanced data learning (Powers, 2011).
Table 2: Results for accuracy, MCC and average(Se, Sp).
Classifier
accuracy
MCC
avg(Se,Sp)
ZeroR
0.765
0.000
0.500
PART
0.710
0.185
0.592
MultilayerPerceptron
0.710
0.199
0.601
J48
0.698
0.118
0.556
RandomForest
0.765
0.161
0.546
BayesNet
0.710
0.185
0.592
NaiveBayes
0.698
0.210
0.611
Logistic
0.716
0.132
0.559
DEQAR-C
0.772
0.358
0.677
The results for the measures associated with a
particular class (precision, recall and F-measure) are
shown in Tables 3 and 4. DEQAR-C did not obtain
the best F-measure result for negative toxicity (the
majority class); but its result was close to the best,
and obtained the best precision. Regarding the
positive toxicity, DEQAR-C obtained the best
precision, recall and F-measure; the classifier
NaiveBayes was the second best. As can be seen, the
F-measure for this minority class was not very high
in all the classifiers, and only DEQAR-C surpassed
the value 0.5.
Table 3: Results for precision, recall and F-measure
(toxicity '+').
Classifier
Precision
Recall
F-Measure
ZeroR
0.000
0.000
0.000
PART
0.378
0.368
0.373
MultilayerPerceptron
0.385
0.395
0.390
J48
0.333
0.289
0.310
RandomForest
0.500
0.132
0.208
BayesNet
0.378
0.368
0.373
NaiveBayes
0.378
0.447
0.410
Logistic
0.357
0.263
0.303
DEQAR-C
0.514
0.500
0.507
A Rule-based Method Applied to the Imbalanced Classification of Radiation Toxicity
151

Figure 3: Results for accuracy, MCC and average(Se, Sp).
Table 3: Results for precision, recall and F-measure
(toxicity '+').
Classifier
Precision
Recall
F-Measure
ZeroR
0.000
0.000
0.000
PART
0.378
0.368
0.373
MultilayerPerceptron
0.385
0.395
0.390
J48
0.333
0.289
0.310
RandomForest
0.500
0.132
0.208
BayesNet
0.378
0.368
0.373
NaiveBayes
0.378
0.447
0.410
Logistic
0.357
0.263
0.303
DEQAR-C
0.514
0.500
0.507
Table 4: Results for precision, recall and F-measure
(toxicity '-').
Classifier
Precision
Recall
F-Measure
ZeroR
0.765
1.000
0.867
PART
0.808
0.815
0.811
MultilayerPerceptron
0.813
0.806
0.810
J48
0.791
0.823
0.806
RandomForest
0.783
0.960
0.862
BayesNet
0.808
0.815
0.811
NaiveBayes
0.821
0.774
0.797
Logistic
0.791
0.855
0.822
DEQAR-C
0.848
0.855
0.851
The results for the measures associated with a
particular class (precision, recall and F-measure) are
shown in Tables 3 and 4. DEQAR-C did not obtain
the best F-measure result for negative toxicity (the
majority class); but its result was close to the best,
and obtained the best precision. Regarding the
positive toxicity, DEQAR-C obtained the best
precision, recall and F-measure; the classifier
NaiveBayes was the second best. As can be seen, the
F-measure for this minority class was not very high
in all the classifiers, and only DEQAR-C surpassed
the value 0.5.
4.2 Prediction Model
After testing and comparing the described classifiers
by stratified cross-validation, the full dataset was
used to obtain a prediction model for the GU
toxicity. DEQAR-C was executed with the same
parameters that achieved the best results in cross-
validation (minsens = 0.7, delta = 0.05, maxAttr = 2,
maxrules = 3). The parameters minsens, maxAttr and
delta are used in the rules generation phase,
controlling the search for rules and the conditions
for the numerical variables (Domínguez et al.,
2015). After this execution, three rules were selected
(see Table 5) and the default class was set to '-'.
As can be seen, the rules are simple with two
variables, because of the constraint due to the
parameter maxAttr (maximum number of variables
in the antecedent).
The combination of these three rules achieves a
covering of 76% of the cases, and from the 39
remaining cases, 28 are negative ones.
The simplicity of the obtained classifier also
makes it more interpretable. It can be easily
analyzed to discover the conditions most likely to be
of influence in the toxicity effects, in contrast with
the greater complexity of other models such as
artificial neural networks or Random Forest. As an
example, Figure 4 shows some of the 24 rules
HEALTHINF 2018 - 11th International Conference on Health Informatics
152
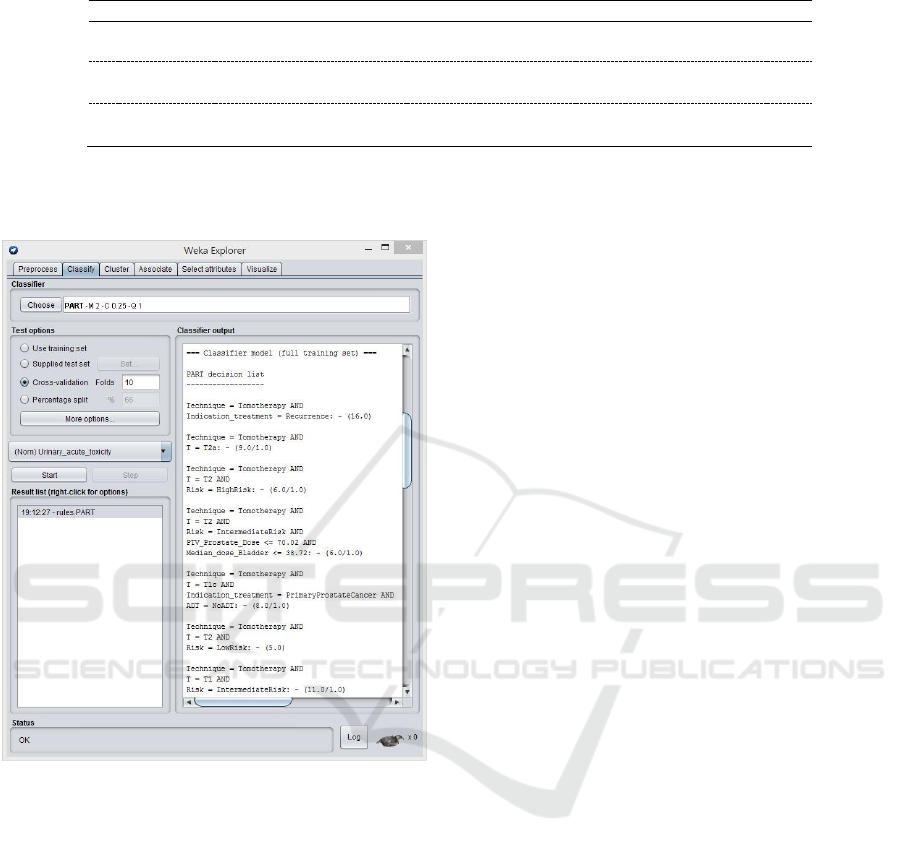
Table 5: Rules obtained in the final classifier.
Antecedent
GU acute toxicity
Technique = Tomotherapy AND Planning tumor volume ≤ 218.62
-
Prostate radiation dose ≥ 70.02 AND Bladder volume ≥ 63.67
+
Technique = Tomotherapy AND Fractionation ≤ 2.52
-
obtained after executing the classifier PART (some
of them with five conditions).
Figure 4: Rules obtained by PART classifier.
4.3 Application to Clinical Practice
As can be seen by analyzing the rules in Table 5,
five of the sixteen independent variables in the
dataset are employed in the model: Technique,
Planning tumor volume, Prostate radiation dose,
Bladder volume and Fractionation. They can be
considered of great relevance in this model for GU
toxicity, because the predictions mainly depend on
their values. Also, some relative importance could
be established between them, because there exists an
ordering in the rules and the search for a match
follows this order.
These variables and their associated values could
be seen as risk factors for GU toxicity. These risk
factors are mostly confirmed by the literature
(Acevedo-Henao et al., 2014); (Ahmed et al., 2013);
(Aizer et al., 2011); (Lopez et al., 2013), which may
corroborate the value of the method employed.
Better stratification of patients based on their
own expected tumor and normal tissue factors will
enable therapy to be highly tailored. Prostate cancer
patients with low-risk toxicity (e.g., men treated
with Tomotherapy and having a lower prostate
volume) might be able to receive a more intense
treatment. Additionally, we can better define the
individual patient subgroups that benefit from
specific components of radiation therapy.
5 CONCLUSIONS
In this work we have presented the application of
several predictive methods to data regarding the
toxicity of radiation therapy for prostate cancer. This
dataset exhibits some imbalance in the classes, with
a 3.3:1 ratio of negative/positive cases.
A rule-based classifier (DEQAR-C) was
described, which works without discretizing the
numerical variables and by selecting a subset of the
best rules extracted for each class. This method was
compared to other classifiers by using cross-
validation with several evaluation measures.
DEQAR-C produced outstanding results in this
classification task, with higher prediction
performance in both classes than the rest of
classifiers. Therefore, it was employed to obtain a
predictive model using the full data. The simplicity
of the model (three rules with two variables) also
makes it more interpretable, which may be useful in
obtaining knowledge from medical data and
subsequently applying it into the clinical practice.
As future work, it would be interesting to test the
proposed approach in another real classification
problem or simultaneously with more datasets.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Ignacio
Azinovic for his support with the employed data.
A Rule-based Method Applied to the Imbalanced Classification of Radiation Toxicity
153

The research presented in this paper was partially
funded by the Regional Government of Andalusia
(Junta de Andalucía) under grant number TIC-7629
and Spanish Ministry of Education and Science
(Grant Number: TIN2009-14057-C03-03).
REFERENCES
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J.,
Rosso, S., Coebergh, J.W.W., Comber, H., Forman, D.
and Bray, F., 2013. Cancer incidence and mortality
patterns in Europe: estimates for 40 countries in 2012.
European Journal of Cancer 49(6), 1374-1403.
American Cancer Society, September 2014.
http://www.cancer.org/cancer/
prostatecancer/detailedguide/prostate-cancer-key-
statistics.
Valeriani, M., Carnevale, A., Osti, M.F., de Sanctis, V.,
Agolli, L. and Enrici, R.M., 2013. Hypofractionated
intensity-modulated simultaneous integrated boost and
image-guided radiotherapy in the treatment of high-
risk prostate cancer patients: a preliminary report on
acute toxicity. Tumori 99(4), 474-479.
Morimoto, M., Yoshioka, Y., Konishi, K., Isohashi, F.,
Takahashi, Y., Ogata, T., Koizumi, M., Teshima, T.,
Bijl, H.P., van der Schaaf, A., Langendijk, J.A. and
Ogawa, K., 2014. Comparison of acute and subacute
genitourinary and gastrointestinal adverse events of
radiotherapy for prostate cancer using intensity-
modulated radiation therapy, three-dimensional
conformal radiation therapy, permanent implant
brachytherapy and high-dose-rate brachytherapy.
Tumori 100(3), 265-271.
Agrawal, R., Imielinski, T. and Swami, A., 1993. Mining
Association Rules between Sets of Items in Large
Databases. In Proceedings of ACM SIGMOD ICMD,
207-216.
Rudin, C., Letham, B. and Madigan, D., 2013. Learning
theory analysis for association rules and sequential
event prediction. Journal of Machine Learning
Research, 14:3441-3492.
Wrobel, S., 1997. An algorithm for multi-relational
discovery of subgroups. Principles of data mining and
knowledge discovery, 78-87.
Gamberger, D., Lavrac, N. and Krstacic, G., 2003. Active
Subgroup Mining: A Case Study in Coronary Heart
Disease Risk Group Detection. Artificial Intelligence
in Medicine 28, 27–57.
Domínguez-Olmedo, J. L., Mata, J. and Pachón, V., 2015.
Deterministic Extraction of Compact Sets of Rules for
Subgroup Discovery. In Proceedings of Intelligent
Data Engineering and Automated Learning – IDEAL,
138-145.
Hastie, T., Tibshirani, R. and Friedman, J., 2009. The
Elements of Statistical Learning: Data Mining,
Inference, and Prediction. Springer-Verlag.
Golhar, M., Iwahori, Y., K. Bhuyan, M., Funahashi, K.
and Kasugai, K., 2017. A Robust Method for Blood
Vessel Extraction in Endoscopic Images with SVM-
based Scene Classification. In Proceedings of the 6th
International Conference on Pattern Recognition
Applications and Methods: ICPRAM, 148-156.
Cortés, X., Serratosa, F. and Moreno-García, C., 2015. An
Interactive Model for Structural Pattern Recognition
based on the Bayes Classifier. In Proceedings of the
International Conference on Pattern Recognition
Applications and Methods: ICPRAM, 240-247.
Liu, B., Hsu, W. and Ma, Y., 1998. Integrating
classification and association rule mining. In
Proceedings of Fourth International Conference on
Knowledge Discovery and Data Mining.
Sun, Y., Wong, A.K.C., Kamel, M.S., 2009. Classification
of imbalanced data: a review. International Journal of
Pattern Recognition and Artificial Intelligence 23(4),
687–719.
Rastgoo, M., Lemaitre, G., Massich, J., Morel, O.,
Marzani, F., Garcia, R. and Meriaudeau, F.,
2016. Tackling the Problem of Data Imbalancing for
Melanoma Classification . In Proceedings of the 9th
International Joint Conference on Biomedical
Engineering Systems and Technologies:
BIOIMAGING (BIOSTEC).
Domínguez-Olmedo, J. L., Mata, J., Pachón, V. and Maña,
M., 2011. A deterministic approach to association rule
mining without attribute discretization. In Proceedings
of the International Conference on Digital Information
Processing and Communications, 188, 140–150.
Domínguez-Olmedo, J. L., Mata, J., Pachón, V. and Maña,
M., 2012. Rule extraction from medical data without
discretization of numerical attributes. In Proceedings
of the International Conference on Health Informatics
(HEALTHINF), 397-400.
Domínguez-Olmedo, J.L. and Mata, J., 2016. Comparison
of Standard Discretization with a New Method for
Quantitative Association Rules. IEEE Latin America
Transactions 14(4), 1879-1885.
Grosskreutz, H. and Ruping, S., 2009. On Subgroup
Discovery in Numerical Domains. Data Mining and
Knowledge Discovery 19(2), 210–226.
Frank, E. and Witten, I. H., 1998. Generating Accurate
Rule Sets without Global Optimization. In
Proceedings of the Fifteenth International Conference
on Machine Learning, 144-151.
Rumelhart, D. E., Hinton, G. E. and Williams, R. J., 1986.
Parallel distributed processing: explorations in the
microstructure of cognition. MIT Press Cambridge,
318-362.
Quinlan, R., 1993. C4.5: Programs for Machine Learning.
Morgan Kaufmann Publishers, San Mateo.
Breiman, L., 2001. Random Forests. Machine Learning
45(1), 5-32.
Pearl, J., 1985. Bayesian Networks: A Model of Self-
Activated Memory for Evidential Reasoning. UCLA
Computer Science Department.
John, G. H. and Langley, P., 1995. Estimating Continuous
Distributions in Bayesian Classifiers. In Proceedings
of the Eleventh Conference on Uncertainty in Artificial
Intelligence, 338-345.
HEALTHINF 2018 - 11th International Conference on Health Informatics
154

Le-Cessie, S. and van Houwelingen, J. C., 1992. Ridge
Estimators in Logistic Regression. Applied Statistics
41(1), 191-201.
Lopez Guerra, J. L., Matute R, Puebla F, Sánchez-Reyes
A, Pontes B, Rubio C, Nepomuceno I, Acevedo C, Isa
N, Lengua R, Praena-Fernandez JM, del Campo ER,
Ortiz M. J and Azinovic I., 2015. Ethnic difference in
risk of toxicity in prostate cancer patients treated with
dynamic arc radiation therapy. Tumori 101(4), 461-8.
Matthews, B. W., 1975. Comparison of the predicted and
observed secondary structure of T4 phage lysozyme.
Biochimica et Biophysica Acta (BBA)-Protein
Structure 405(2), 442–451.
Arlot, S. and Celisse, A., 2010. A survey of cross-
validation procedures for model selection. Statistics
Surveys, 40-79.
Frank, E., Hall, M. A. and Witten, I. H., 2016. Data
Mining: Practical Machine Learning Tools and
Techniques. Morgan Kaufmann.
Powers, D. M. W., 2011. Evaluation: from precision,
recall and F-measure to ROC, informedness,
markedness and correlation. International Journal of
Machine Learning Technology 2(1), 37-63.
Acevedo-Henao, C. M., Lopez, J. L., Matute, R. and
Azinovic, I., 2014. Image-guided radiation therapy
based on helical tomotherapy in prostate cancer:
minimizing toxicity. Oncology Research and
Treatment 37(6), 324-30.
Ahmed, A. A., Egleston, B., Alcantara, P., Li, L., Pollack,
A., Horwitz, E.M. and Buyyounouski, M. K., 2013.
A novel method for predicting late genitourinary
toxicity after prostate radiation therapy and the need
for age-based risk-adapted dose constraints.
International Journal of Radiation Oncology, Biology,
Physics, 86(4), 709-15.
Aizer, A. A., Anderson, N. S., Oh, S. C., Yu, J. B.,
McKeon, A. M., Decker, R. H. and Peschel, R. E.,
2011. The impact of pretreatment prostate volume on
severe acute genitourinary toxicity in prostate cancer
patients treated with intensity-modulated radiation
therapy. International Journal of Radiation Oncology,
Biology, Physics, 79(2), 379-84.
Lopez, J. L., Isa, N., Matute, R., Russo, M., Puebla, F. and
Miran, K. M., 2013. Hypofractionated helical
tomotherapy using 2.5-2.6 Gy daily fractions for
localized prostate cancer. Clinical and Translational
Oncology 15(4), 271-7.
A Rule-based Method Applied to the Imbalanced Classification of Radiation Toxicity
155
