
A General Framework for the Distributed Management of Exceptions
and Comorbidities
Alessio Bottrighi, Luca Piovesan and Paolo Terenziani
DISIT, Computer Science Institute, Universit
`
a del Piemonte Orientale, Italy
Keywords:
Computer Interpretable Guideline (CIG), Concurrent Execution of Multiple CIGs, CIG-exception Handler,
System Architecture.
Abstract:
In the last decades, many different computer-assisted management systems for Computer Interpretable Guide-
lines (CIGs) have been developed. While CIGs propose a “standard” evidence-based treatments of “typical”
patients, exceptions may arise, as well the need to cope with comorbidities. The treatment of deviation from
“standard” execution has attracted a lot of attention in the recent literature, but the approaches proposed are
focused on the treatment either of exceptions or of comorbities. However, this is a clear limitation, since du-
ring a CIG execution, both these issues can occur. In this paper, we propose the first approach which supports
the integrated treatment of both exceptions and comorbidities. To achieve such a goal, we propose a modular
client-server architecture supporting the concurrent execution of multiple guidelines. The architecture propo-
sed has been designed as a further layer building upon “traditional” execution engines for a single CIG. Thus,
our methodology is general and can be used to extend the CIG systems in the literature. Finally, we describe
our approach in action on a case study, in which a comorbid patient is treated for Peptic Ulcer and for deep
Venous Thrombosis and, during the treatment, she manifests a heart failure.
1 INTRODUCTION
Clinical Practice Guidelines (CPGs) represent the cur-
rent understanding of the best clinical practice. CPGs
are gaining a major role to improve the quality and to
reduce the cost of health care. The ICT technology
can further enhance the impact of CPGs. Many dif-
ferent systems have been developed to manage Com-
puter Interpretable clinical practice Guidelines (CIGs
for short). Such approaches are characterized by a
specifc formalism to represent CPGs. CIG forma-
lisms are usually based on a Task Network Model: a
(hierarchical) model of the CPG control flow as a net-
work of specific tasks. Such formalisms are “formal”
and allow one to unambiguously represent guideline
procedures and recommendations. Besides suppor-
ting formal languages to acquire and represent CPGs,
CIG systems usually also provide execution engines
that allow user physicians to “instantiate” general gui-
delines on specific patients: by accessing the patient
clinical data, the execution engine shows to the user
physicians only those paths of actions that are ap-
plicable to the patient at hand. In such a way, they
provide patient-oriented recommendations to physici-
ans, allowing them to fulfill the gap between the CPG
generality and the specificity of the patient at hand.
A survey and/or a comparative analysis of these sys-
tems is outside the goals of this paper, but a compari-
son of Asbru, EON, GLIF, Guide, PROforma, PRO-
DIGY can be found in (Peleg et al., 2003). Bottrighi
et al. (Bottrighi et al., 2009) extends it to consider
also GLARE and GPROVE. (Peleg, 2013; Anselma
et al., 2015) are recent surveys of the state-of-the-art.
One of the main goals of CPGs and CIGs is to cap-
ture medical evidence and to put it into practice. Ho-
wever, from one side, evidence is essentially a form
of statistical knowledge, and it is used to capture the
generalities of classes of patients, rather than the pe-
culiarities of a specific patient. From the other side,
demanding to expert committees the elicitation of all
possible executions of a CPG on any possible specific
patient in any possible clinical condition is an infea-
sible task. Thus, several conditions are usually impli-
citly assumed by experts building a CPG: (i) ideal pa-
tients, i.e., patients that have only the disease conside-
red in the CPG (thus excluding the concurrent appli-
cation of more than one CIG), and (ii) “statistically re-
levant” patients not presenting rare peculiarities/side-
effects; (iii) ideal context of execution, so that all ne-
cessary resources are available. However, when a spe-
cific physician applies a given CIG to a specific pa-
tient, unexpected conditions may show up. Such si-
66
Bottrighi, A., Piovesan, L. and Terenziani, P.
A General Framework for the Distributed Management of Exceptions and Comorbidities.
DOI: 10.5220/0006552800660076
In Proceedings of the 11th Inter national Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 5: HEALTHINF, pages 66-76
ISBN: 978-989-758-281-3
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

tuations are unexpected, and, as such, cannot be spe-
cified a priory in the CIGs. However, especially in
case of unexpected life threatening problems, the phy-
sician must start soon to cope with the new problems
(possibly suspending or ending the “standard” execu-
tion of the current CIG, or concurrently with it). Such
problems have been usually indicated with the term
“exceptions” within the CIG community, since they
are exceptions with respect to the “standard” execu-
tion of a CIG.
Another challenging problem that might involve
deviations from the “standard” execution of a CIG is
the treatment of comorbid patients. The problem is
that, by definition, CIGs address specific clinical ci-
rcumstances (i.e., specific pathologies), and, unfortu-
nately, in comorbid patients the treatments of single
pathologies may dangerously interact with each ot-
her. Also, the approach of proposing an ad-hoc “com-
bined” CIG to cope with each possible comorbidity
does not scale up (Michalowski et al., 2013). For
these reasons, new methodologies have been recently
introduced to manage multiple CIGs on comorbid pa-
tients (see, e.g., the survey in (Fraccaro et al., 2015)).
1.1 Related Work
Within the CIG community, the “exceptions” and co-
morbidities have been always managed in isolation.
Several frameworks have been already proposed
to cope with “exceptions” (see, e.g. (Fox et al., 1998;
Leonardi et al., 2012; Tu and Musen, 1999; Grando
et al., 2010; Quaglini et al., 2001; Peleg et al., 2009)).
In most of such approaches, the “standard” executor
of a CIG is extended with some mechanism to trig-
ger exceptions (on the basis of the patient’s data) and
to activate their treatment, synchronizing such a treat-
ment with the execution of the current CIG. Different
mechanisms of synchronization have been proposed.
On the other hand, a range of different techni-
cal solutions have been proposed to cope with co-
morbidities, spanning from the use of constraint lo-
gic programming (Michalowski et al., 2013; Wilk
et al., 2017) to answer set programming (Merhej et al.,
2016), from rules (L
´
opez-Vallverd
´
u et al., 2013) to
agents (S
´
anchez-Garz
´
on et al., 2013). Notably, some
of such approaches focus on the automatic genera-
tion of a unique “merged” CIG avoiding the undesi-
red CIG interactions (consider, e.g. (L
´
opez-Vallverd
´
u
et al., 2013; S
´
anchez-Garz
´
on et al., 2013)). In such a
way, a “standard” CIG executor can be used to enact
the “merged” CIG. However, in the clinical practice,
(1) there are usually different ways to manage inte-
ractions, and physicians want and must (for ethical
reasons) be the protagonists of such a decision. And,
more importantly for the current work, (2) though
in clinical practice the interactions between CIGs are
managed, physicians do not look at the solution as a
single “merged” CIG: they still look at the treatment
of a comorbid patient as the concurrent execution of
multiple CIGs.
In conclusion, there are several approaches mana-
ging either “exceptions” or comorbidities, but no one
provides a integrated support of them. This is a ma-
jor and clear limitation to their application in practice,
also given the occurrence frequency of the above phe-
nomena. For instance, several studies demonstrate a
prevalence of comorbidities on an average of 25% of
the population, ranging from 10% (in younger people)
to 78% (in older people) (van den Akker et al., 1998;
Barnett et al., 2012). Thus, we aim at fulfilling the
gap between the support provided by the current CIG
systems and real world requirements.
1.2 Goals and Original Contributions
Until now, within the CIG literature, exceptions and
comorbidities have been treated as separate pheno-
mena
1
, so that current approaches cope either with
exceptions, or with comorbidities. This is a clear li-
mitation of the state of the art, since both phenomena
may co-occur on specific patients. In this paper, we
first propose a CIG approach facing both phenomena,
thus overcoming such a relevant limitation of the cur-
rent literature. Additionally, unlike (L
´
opez-Vallverd
´
u
et al., 2013; S
´
anchez-Garz
´
on et al., 2013), we aim at
maintaining the distinction between CIGs, even in the
case of comorbid patients.
Our goal is to propose the first general framework
that copes with both exceptions and comorbidities.
The starting point of our approach is that the mana-
gement of patients affected by multiple problems re-
quires
(i) A support for the concurrent and distributed
(i.e., carried on by different agents) execution of
CIGs, and to synchronize them. In the case of
comorbid patients, it will support the execution
of one CIG for each one of the patient’s disea-
ses; in the case of an “exception”, it will support
the execution of the original CIG plus the plan
1
This choice is, in our opinion, quite surprising, since
there does not seem to be a clear cut between the two pheno-
mena. Just as one prototypical example, in (Leonardi et al.,
2012) heart failure is considered as an “exception” for a pa-
tient treated with a CIG for trauma. But, when a patient with
a trauma manifests a heart failure, s/he becomes a comorbid
patient, and attention must be paid to avoid dangerous inte-
ractions between the treatment (CIG) for the trauma and the
treatment (CIG) for the hearth failure.
A General Framework for the Distributed Management of Exceptions and Comorbidities
67

(which can be formalized as a CIG) to manage
the exception.
(ii) A support for detecting the possible interacti-
ons between such concurrent CIGs, and for ma-
naging them (avoiding dangerous interactions)
(iii) A support for detecting new patient’s problems
(i.e., changes in the status of the patient that re-
quire new treatments – thus, new CIGs).
In the rest of the paper, we propose the first CIG
framework providing all such supports in an integra-
ted way. While (1)-(3) are the main goals of our
approach, we also take into account a further, more
“technical” goal. The specialized literature proposes
several “consolidated” execution engines, to execute a
CIG for a specific patient. Many of such approaches
(including GLARE and its recent extension META-
GLARE (Terenziani et al., 2014; Bottrighi and Te-
renziani, 2016) have achieved good results, provi-
ding physicians with friendly environments to execute
CIGs. We think that it would really be a pity to waste
such an amount of good work, building from scratch
a new concurrent execution engine. Thus, an addi-
tional main goal of our approach is that of devising
a modular approach for the concurrent execution, in
which the execution engine of a CIG in isolation is
maintained, and it is extended and integrated in a ge-
neral framework supporting synchronization and con-
currency. Notably, although we are building our fra-
mework on top of META-GLARE (in the sense that
each “Exec” module - see Fig.3 below - is an instanti-
ation of META-GLARE execution module), our met-
hodology is general and can be adapted for similar
CIG systems (such as, e.g., (Fox et al., 1998; Shahar
et al., 1998)).
Furthermore, notice that the framework we have
developed also supports the fact that multiple healt-
hcare agents may be involved in the execution of each
single CIG for a specific patient. We cope with multi-
ple agents using the methodology in (Bottrighi et al.,
2013) and generalizing it to the context of multiple
CIGs. For the sake of brevity, such a topic is no furt-
her discussed within this paper.
Notably, the main contribution of this paper is
the definition of (the architecture of) a system-
independent framework for the distributed manage-
ment of exceptions and comorbidities. We are cur-
rently implementing a prototype of the framework on
top of META-GLARE and of its module for mana-
ging the interactions (Anselma et al., 2017).
2 A GENERAL VIEW OF THE
BEHAVIOR OF OUR
FRAMEWORK
While the architecture of our framework is proposed
in Section 3, here we informally discuss the basic
data/knowledge sources (ovals in Fig. 1) managed by
our framework, and its general behavior (see Fig. 1).
First, though in the literature (see, e.g., (Fox et al.,
1998; Grando et al., 2010; Leonardi et al., 2012; Pe-
leg et al., 2009; Quaglini et al., 2001; Tu and Musen,
1999)) different types of exceptions have been identi-
fied, for the sake of brevity in this paper we focus only
on the most “common” ones, i.e., on the exceptions
arising because of unexpected changes in the status
of the patient, requiring a (new) treatment (i.e., with
CIG-independent patient-exceptions, in the termino-
logy in (Leonardi et al., 2012)).
Second, it is important to clarify that there is a
main “practical” difference between the management
of exceptions and the one of interactions (e.g., the in-
teractions that may arise between actions of different
CIGs operating on the same patient). Exceptions can-
not be avoided: the status of the patient has already
changed, and an exception arises because of such a
change (i.e., it is triggered by the new status of the
patient). Moreover, of course, the exception must be
managed, usually by starting a new treatment (which,
indeed, may be represented by a CIG) for it.
In our approach, the treatment of exceptions is
modeled by a KB (called “Exception KB”; see
Fig. 1) consisting of triggering rules of the form
hCondition, Managi, where “Condition” indicates a
Boolean condition (called “triggering” conditions) on
the status of the patient, and “Manag” represents the
actions to cope with such a condition (representable
as a new CIG) plus constraints about how such new
actions have to be synchronized with (the execution
of) the current CIG(s). Notably, in our approach, the
“Exception KB” is static, in the sense that the rules
it contains are patient-independent, and are perma-
nently stored.
On the contrary, undesired interactions should be
detected a-priori and avoided (through some manage-
ment operation). META-GLARE, for instance, is pro-
vided with a framework (see the Interaction Analysis
module in Fig. 1; rectangles represent computatio-
nal modules) for (i) supporting physicians in the fo-
cusing on specific parts of the CIGs
2
(Piovesan et al.,
2
CIGs may consist of hundreds of actions and/or alter-
native paths. An extensive check of all interactions could
provide a combinatorial number of cases, most of which
are not interesting for the patient at hand. Physician-driven
focusing is an essential step to avoid an unnecessary com-
HEALTHINF 2018 - 11th International Conference on Health Informatics
68
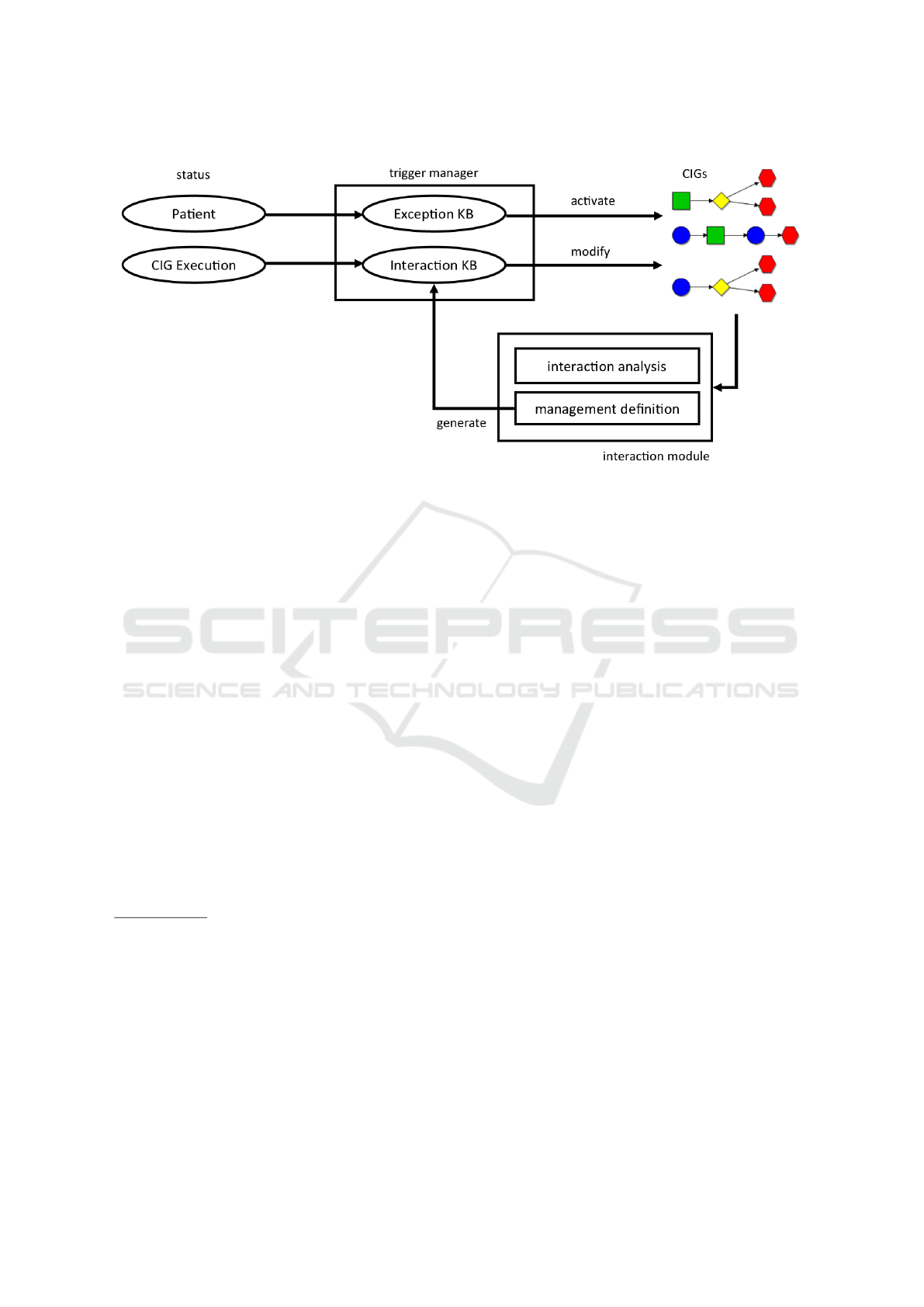
Figure 1: Graphical representation of our treatment of exceptions and interactions.
2015), (ii) automatically detecting (based on CIG-
independent ontological knowledge) the interactions
between the focused action (Piovesan et al., 2014), as
well as a suite of management options (derived from
the medical literature) to manage them (e.g., avoiding
them by delaying some actions, or managing them
through local modifications of the involved CIGs (Pi-
ovesan and Terenziani, 2015); see the Management
Definition module in Fig. 1). Although the analysis
of interaction should be performed a priori, generally
the management option chosen by physicians has not
to be enacted soon. Indeed, it had to be enacted only if
and when, during the execution of the CIGs, the con-
ditions identifying the onset of the interactions arise
3
.
As a consequence, the treatment of interactions may
be modelled by a KB (called “Interaction KB”; see
Fig. 1) containing hCondition, Managi pairs. Howe-
ver, differently from the rules for exceptions discus-
sed above, here:
(i) triggering conditions have as input the status of
execution of the CIGs
(ii) “Manag” indicates the operations to implement
binatorial explosion of the computation and of the number
of the identified interactions.
3
As an example, a possible undesired interaction bet-
ween the actions Act1 in CIG
A
and Act2 in CIG
B
can be
detected and physician can choose to manage it via the sub-
stitution of Act2 with a set of actions achieving the goal of
Act2, but non-interacting with Act1. However, such a sub-
stitution must be performed only in case the execution of
the two CIGs enforces the execution of both Act1 and Act2
(at times such that their effects may overlap in time). In-
deed, if in CIG
A
a path of actions not including Act1 has
been selected for execution, there is no need to substitute
Act2.
the management option chosen to cope with an
interaction (e.g., operations to locally modify a
CIG)
The “Interaction KB” is dynamic: the system dyna-
mically add rules into it whenever a new interaction
has been detected and a management for it has been
chosen, and it deletes such rules when they are not
useful any more. Indeed, there would be no reason to
permanently store such rules, since they are specific
to the execution of a set of CIGs for a specific patient.
In Fig. 1, we show that a unique manager, the
“Trigger Manager” can uniformly operate on both
types of rules. Fig. 1 also shows that the output of
the execution of an “Exception” rule may be the acti-
vation of a new CIG, while the result of the execution
of an “Interaction” rule may usually be a modifica-
tion of some CIGs. It also shows that, when multiple
CIGs are active, the Interaction Analysis module may
be used to analyse possible interactions, and the Ma-
nagement Definition module can support the manage-
ment of such interactions. The output of the Manage-
ment Definition module is a new hCondition, Managi
rule, dynamically stored in the Interaction KB.
While Fig. 1 graphically illustrates the behaviour
of our framework, in Section 3 we discuss the archi-
tecture we have identified to achieve it, and conside-
ring the fact that the management of exceptions and
interactions may involve forms of synchronizations
between the different CIGs (possible synchronizati-
ons between CIGs have been omitted, for the sake of
clarity, from Fig. 1).
A General Framework for the Distributed Management of Exceptions and Comorbidities
69
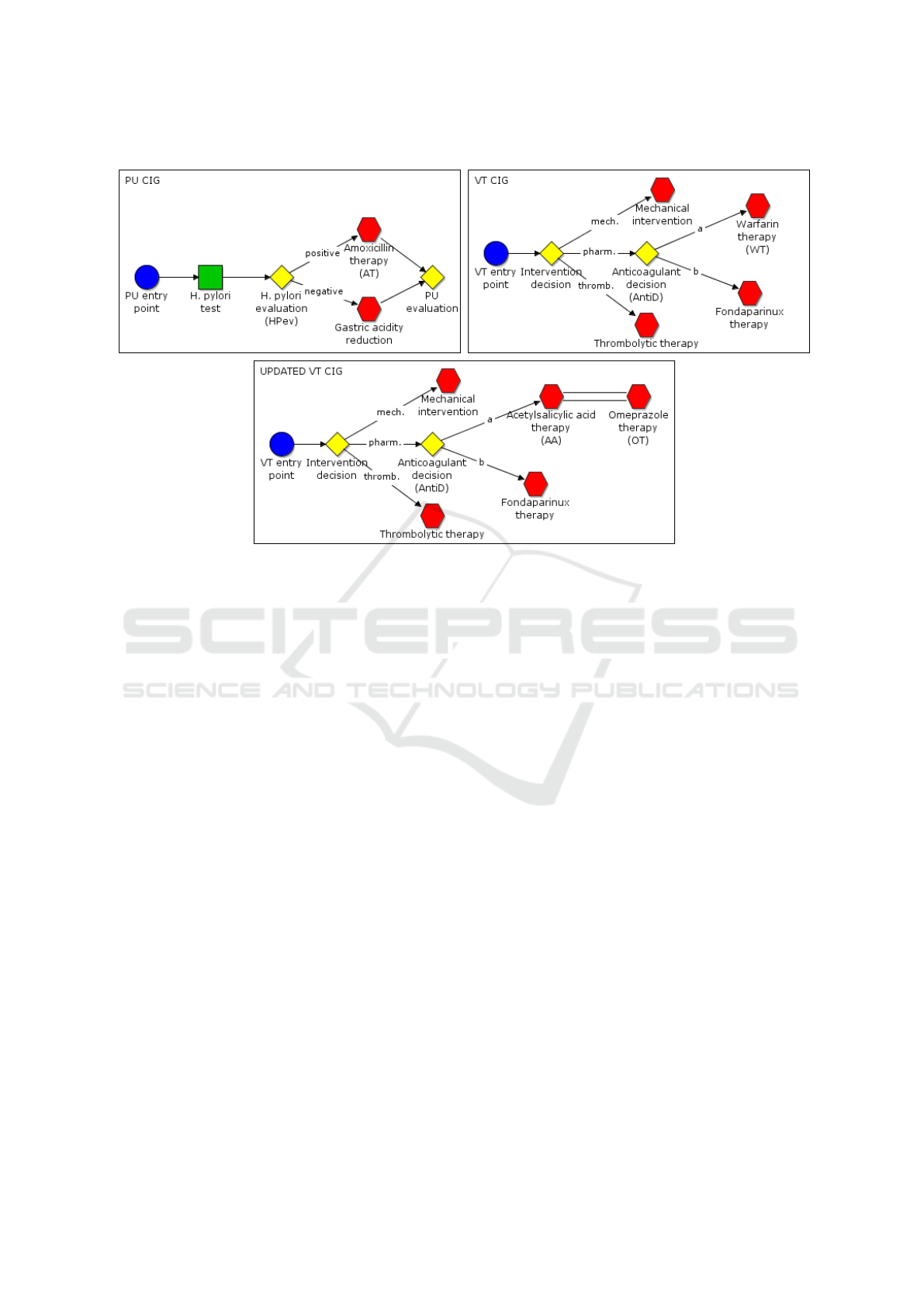
Figure 2: Part of PU and VT original CIGs (above) and the updated version of VT after the managing of the interaction
(below).
2.1 Case Study
In this section, we present a “synthesized” case study,
which has been created with the help of some phy-
sicians of Azienda Ospedaliera “San Giovanni Bat-
tista” in Turin in order to be able to exemplify the
main features of our approach. We consider a comor-
bid patient, who is treated for Peptic Ulcer (PU) and
for deep Venous Thrombosis (VT) and has a heart fai-
lure (i.e., an “exception” arises) during the execution
of these CIGs. The two diseases are managed by two
specific CIGs (the upper part of Fig. 2 shows simpli-
fied parts of the CIGs). Besides the CIGs, additional
medical knowledge is available, including the trigger
for exceptions. In our example, among them, we con-
sider the exception for heart failure (notably, in this
context, heart failure can be considered an exception:
it is not statistically recurrent in PU and VT, thus its
treatment is not contained into the original CIGs).
In our example, the CIGs for PU and VT are
executed concurrently by two different physicians:
Physician
1
manages PU, and Physician
2
manages VT.
We consider a sample working section articulated as
follows.
Step 1. We suppose that Physician
1
, at a certain point
of execution of PU and VT (e.g., at the beginning),
decides to analyze the possible interactions between
the two CIGs. To do so, Physician
1
exploits the Inte-
raction Manager module (see Fig. 1) focusing on re-
levant parts of PU and of VT, and the Interaction Ma-
nager module detects an interaction between warfarin
therapy (WT) in VT and amoxicillin therapy (AT) in
PU.
Step 2. Physician
1
chooses to manage such an inte-
raction replacing the action WT with an alternative
plan, having the same goal. For instance, the new
therapeutic plan may be the combination of acetylsa-
licylic acid (AA) therapy and omeprazole (OT) ther-
apy. As we will see in the Section 4, the Interaction
Manager module creates a trigger rule to implement
such a management (if/when required).
Step 3. Physician
1
and Physician
2
go on with the
independent executions of the CIGs. We suppose
that in PU “PU start”, “H.Pylori test”, “HPev” with
exit “positive” has been executed; in the meanwhile,
in VT “VT start”, “intervention decision”, with exit
“pharm”, and “AntiD” with exit “a” has been execu-
ted.
Step 4. At this point, the chosen management of the
interaction is required, and is executed (i.e. the trig-
ger rule created at step 2 above is executed, thus mo-
difying the CIG as shown in the lower part of Fig. 2).
Step 5. The execution continues on the modified
CIGs.
Step 6. To exemplify all the main features of our ap-
proach, we further suppose that at this point the pa-
tient has a heart failure, and we show how our frame-
work supports its treatment.
HEALTHINF 2018 - 11th International Conference on Health Informatics
70

3 ARCHITECTURE FOR THE
CONCURRENT EXECUTION
OF MULTIPLE CIGS
3.1 The Architecture
Our approach is based on the client-server model.
This choice is motivated by the need (i) to support a
distributed execution of the patient treatments, since
different CIGs can be managed by different physici-
ans (i.e. each physician needs a client to manage her
CIGs) and (ii) to have a global vision of patient treat-
ments, and to “synchronize” them (such a vision will
be stored and managed in the server). Notably, from
an abstract point of view our approach can be descri-
bed as an agent based system (i.e. each module can
be seen as an agent).
For sake of simplicity, in this paper we assume
that all the CIGs are related to the same patient. The
extension to cope with more than one patient is obvi-
ous. We propose a server model (see Fig. 3) compo-
sed by the following modules:
• a “General Manager” (in the middle of the “Ser-
ver” in Fig.3): it maintains the global vision of
the patient and of her treatments (i.e. global data
structures). It interacts with the other modules to
update such a vision and to synchronize them (the
functionalities of such a module are described in
more detail in subsection 3.2);
• the “Executor Modules”(“Exec CIG1”, “Exec
CIG2”, and “Exec CIG3”, in the left part of the
“Server” in Fig.3; notably, there is one “Exec”
module for each CIG under execution for the pa-
tient). Each Executor manages the execution of a
CIG for a specific patient (Bottrighi et al., 2015)
(see subsection 3.3);
• the “Interaction Manager” (top right part of the
“Server” in Fig.3): it supports the study of the
interactions between CIGs and defines how they
should be managed (see subsection 3.4);
• the “Trigger Manager” (bottom right part of the
“Server” in Fig.3): it manages the triggers in KBs
(see subsection 3.5).
Notably, the architecture of our framework is open.
It is possible to add the new modules to provide new
facilities, by specifying their communication API (i.e.
how they communicate with the other modules, the
patient’s DB and the client).
The client provides physicians with a GUI to sup-
port the execution of one or more CIGs (e.g. in Fig. 3
Client
1
allows to manage the execution of CIG
1
and
CIG
2
, while Client
2
supports the execution of CIG
3
).
Each client sends/receives messages to/from the exe-
cutor module to manage the execution of the CIGs.
Moreover, physicians can activate the interaction mo-
dule to study possible interactions between two or
more CIGs (e.g. in Fig. 3, Client
1
activates it).
3.2 The General Manager Module
The General Manager is the core of the system, since
it supports the concurrent execution of CIGs on a gi-
ven patient. To achieve such a goal, it manages the
interplay between the other modules in the server by
(i) sending/receiving messages, and (ii) maintaining
two data structures to provide a “global vision” of the
execution of the CIGs: (i) the graph of CIG depen-
dencies and (ii) the yellow pages of CIGs. Such data
structures work as a shared memory, where all the
modules have the read permissions, while the General
Manager has also the write permission.
The graph of CIG dependencies has two compo-
nents: nodes and arcs. Each node represents one CIG
under execution (for the given patient). The arcs re-
present the dependencies between such CIGs. An arc
starting from a node A and ending into a node B me-
ans that B must be suspended by the execution of A.
Thus, the graph represents the synchronization bet-
ween CIGs: CIGs without entering arcs are active,
while CIGs reached by an arc are temporarily sus-
pended. We provide a set of primitives to update the
graph: creation/deletion of a node, creation/deletion
of an arc.
The yellow pages of CIGs store all the instances of
CIGs currently in execution. The operations provided
to update the yellow pages are: add a CIG, remove a
CIG, update a pharmacological dosage, update a tem-
poral constraint, add a node to a CIG, add an arc to a
CIG (assuming that CIGs are represented as a Task-
Network Model).
The updates to the data structures are triggered
by messages sent by the other modules in the server.
A message represents a list of instructions expressed
using the primitives described above. The General
Manager manages messages as transactions, i.e. units
of work performed in an atomic way. It performs all
the updates required and then it notifies such upda-
tes to the modules to maintain the synchronization.
The General Manager manages the message of upda-
tes using a FIFO policy.
A General Framework for the Distributed Management of Exceptions and Comorbidities
71
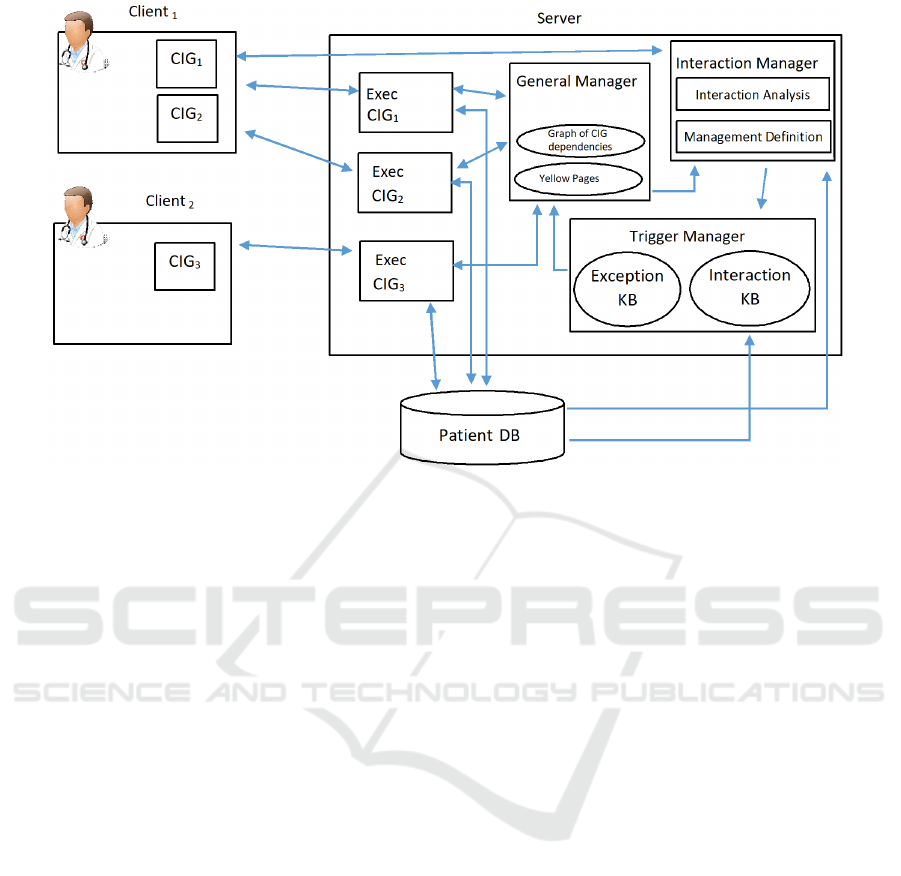
Figure 3: General Architecture of our framework. The arrows show the flow of information between the different modules.
3.3 The Executor Module
The Executor module manages the execution of a
CIG instance for a specific patient. In our appro-
ach, there is an instance of Executor for each CIG
under execution. The Executor of a CIG can be
active or suspended depending on the current state
(i.e. active/suspended) of the CIG represented in the
graph of dependencies. Each instance of Executor re-
ads (i) the instance of the CIG that it has to execute
from the yellow pages, and (ii) the patient data from
the Patient DB. The Executor interacts with a speci-
fic client to execute the current actions in the CIG.
In case the CIG is terminated, the Executor sends a
message to the General Manager, to remove the node
(representing the CIG) from the graph, and remove
the CIG from the yellow pages. Specifically, we use
the executor of META-GLARE (Bottrighi and Teren-
ziani, 2016), but our methodology is mostly system-
independent, and it can be adapted for use any CIG
executor (such as, e.g., (Fox et al., 1998)) or Asbru
(Shahar et al., 1998)).
3.4 The Interaction Manager Module
The Interaction Manager module supports the de-
tection and the definition of management for CIG in-
teractions. It is composed by two modules (see Fig.
3): the Interaction Analysis and the Management
Definition. The Interaction Analysis module (see (Pi-
ovesan et al., 2014)) operates in two steps. First,
it provides physicians with a navigation tool (opera-
ting at the different abstraction levels supported by
the given CIGs) supporting the choice of a specific
part (called “focus”) of the CIGs, the part currently of
interest for the treatment of the current patient. Se-
cond, it provides a knowledge-based tool that auto-
matically detects all the possible interactions between
the actions in the “focus”. Moreover, this module has
been recently extended with a set of facilities to tem-
porally analyze interactions (Anselma et al., 2017),
distinguishing among temporally certain, possible or
impossible interactions and performing hypothetical
reasoning. Once detected an interaction, the Mana-
gement Definition module (Piovesan and Terenziani,
2015) supports physicians in the selection of a ma-
nagement, choosing among different modalities (i.e.,
the management options (Piovesan and Terenziani,
2015)). Notably, in our approach, managements are
not applied immediately to CIGs, but through the cre-
ation of dynamic trigger rules (see the discussion in
Section 2). The triggers have the form hCondition,
Managi where “Condition” indicates a Boolean con-
dition on the execution of specific CIG action(s) or
decision result(s), and “Manag” represents the acti-
ons to cope with such a situation. These actions can
be described using a subset of primitives to operate on
the global data structures (see Section 3.2). Such trig-
ger rules are automatically generated by the by a spe-
cific component of the Management Definition mo-
dule (the “Trigger Generator”, not detailed in Fig.3
for the sake of brevity and clarity). The “Trigger Ge-
HEALTHINF 2018 - 11th International Conference on Health Informatics
72

nerator” takes as input from the other modules the de-
tected interacting actions, and the management opti-
ons chosen to manage such an interaction, plus addi-
tional parameters. The Trigger Generator consists of
a set of parametric procedures, one for each manage-
ment option, to automatically generate a trigger, on
the basis of the input parameters. Then, the trigger is
sent as a message to the Trigger Manager.
For instance in Section 4 we show the trigger crea-
ted by the Interaction Manager module to manage the
interaction between WT and AT (see section 2.1).
3.5 The Trigger Manager
The Trigger Manager module manages the triggers.
To achieve such a goal, it has (i) to check whether the
triggers stored in the KBs fire and then to notify that
the management had to be applied and (ii) to maintain
up-to-date the Interaction KB, since it is a dynamic
KB (see details in Section 2).
To cope with (i), the Trigger Manager evaluates
whether a rule in the KBs had be executed. The form
of rules is hCondition, Managi (see Section 2) and the
Trigger Manager checks whether Condition is true or
not (i.e. the patient status retrieved in the Patient DB
or the execution status of the CIGs retrieved in the
yellow pages satisfy Condition). If Condition is true,
the Trigger Manager sends a message to the General
Manager. Such a message contains Manag (i.e. the
set of instructions to cope with the situation described
in Condition).
To cope with (ii), the Trigger Manager adds a
trigger to the Interaction KB, when it receives a mes-
sage from the Interaction Manager module. Each
message contains a trigger that has to be added. The
Trigger Manager manages the messages using a FIFO
policy.
The triggers in the Interaction KB are not perma-
nent, since they are context and patient dependent.
Thus, the Interaction Manager removes a trigger: (i)
when it is used (in the case that it is not reusable,
e.g. in the case it is applied to a repeatable part of
the CIGs, it is removed when the repetitions of such
a part is ended) or (ii) when one of the CIGs in its
Condition ends.
4 OUR SYSTEM IN ACTION:
MANAGING THE CASE STUDY
We describe how our framework works on the case
study described in subsection 2.1. The patient is af-
fected by both Peptic Ulcer (PU) and deep Venous
Thrombosis (VT) and two CIGs are executed to
treat such diseases. Two physicians are involved:
Physician
1
, managing PU, and Physician
2
, mana-
ging VT. In our framework, each physician interacts
with the system via a client: (1) Physician
1
uses
Client
PU
to execute the CIG PU via the executor in-
stance Executor
PU
, and (2) Physician
1
uses Client
V T
,
to execute the CIG VT via the executor instance
Executor
V T
. Suppose that both physicians are ma-
naging the first action in the CIG (but this is not re-
strictive at all). In such a context, the graph of de-
pendencies contains two independent nodes (one for
PU and one for VT), while the yellow pages contain
the current instances of the CIGs. The Interaction KB
is empty and Exception KB contains the triggers to
manage the exceptions. In our example, among the
others, the trigger TR-HF (i.e. the trigger for heart
failure) is stored in the Exception KB:
TR-HF:
(1) h(Heart Failure = TRUE),
(2) (ADD_NODE HF-PLAN TO GRAPH;
(3) ADD HF-PLAN TO YELLOW PAGES;
(4) ADD_ARC from HF-PLAN to VT;
(5) ADD_ARC from HF-PLAN to PU;)i
In TR-HF, the Condition (line 1) captures that the
patient has a heart failure, the Manag (lines 2-5) des-
cribes the instructions that must be executed to ma-
nage it. In short, (2)-(5) encode the commands to
activate a new CIG “HF-PLAN” suspending the exe-
cution of VT and PU.
To analyze the possible interactions between the
two CIGs, Physician
1
(through Client
PU
) calls the In-
teraction Manager module and selects the relevant
part of CIGs that the module has to analyse (i.e., the
“focus”). The Interaction Manager identifies all the
interaction between the actions in the “focus” via the
Interaction Analysis module. In this specific example,
the Interaction Manager module finds an interaction
between warfarin therapy (WT) and amoxicillin ther-
apy (AT). Such an interaction increases the anticoagu-
lant effect of warfarin and raises the risk of bleedings.
As described in subsection 2.1, Physician
1
decides to
apply the replanning management option (Piovesan
and Terenziani, 2015), substituting WT with an alter-
native new plan. Such a new plan is automatically
generated by the Management Definition module (as
described in (Bottrighi et al., 2016)). In our example,
the new therapeutic plan is the combination of ace-
tylsalicylic acid (AA) therapy and omeprazole (OT)
therapy.
Then the Trigger Generator is invoked. It takes
as input the PU and VT CIGs, the management op-
tion chosen by Physician
1
(i.e., the replanning option)
and the new alternative plan, and (automatically) pro-
duces as output the trigger rule TR-WTAT described
A General Framework for the Distributed Management of Exceptions and Comorbidities
73

below. The Condition part of TR-WTAT represents
the conditions under which the interaction can occur.
In particular, in our example, AT and WT interacts in
case (line 1): (i) the decision AntiD has been taken,
having as result to execute the path “a” which con-
tains WT, and (ii) either the decision HPev has been
taken with result “positive” (i.e. choosing the path
containing AT), or HPev has not been already exe-
cuted (in this last situation, the system preventively
applies the management, avoiding the cases in which
decision HPev is taken with “positive” result only af-
ter WT has been executed, impeding the application
of the management). Notably, such a condition is au-
tomatically built by the Trigger Generator, through a
navigation throughout the PU and VT CIGs. The Ma-
nag part of TR-WTAT is automatically built by the
Trigger Generator on the basis of the management
option chosen by Physician
1
and the new alternative
plan. Specifically, the Manag part of TR-WTAT pres-
cribes to (line 2) remove WT, and (lines 3-4) to add
AA, OT and (lines 5-6) the corresponding arcs in the
CIG VT (the result of the execution of TR-WTAT is
shown in Fig. 2).
TR-WTAT:
(1) h(Exec(AntiD)=a AND (Exec(HPev)=positive
OR NOT Exec(HPev)),
(2) (remove action WT in VT;
(3) ADD_ACTION AA to VT;
(4) ADD_ACTION OT to VT;
(5) ADD_ARC in VT from AntiD to AA;
(6) ADD_ARC in VT from AA to OT;)i
Then, the Interaction Manager module sends a
message containing the TR-WTAT rule to the Trigger
Manager.
As a consequence, the Trigger Manager adds it to
the Interaction KB (see Fig. 2). Then, the two physi-
cians can independently go on with the execution of
the CIGs.
For instance, suppose that Physician
1
(through
Client
PU
) has executed the actions “PU start”,
“H.Pylori test”, and “HPev”, which results positive; in
the meanwhile, Physician
2
(through client Client
V T
)
has executed “VT start”, “intervention decision”, with
exit “pharm”, and “AntiD” with exit “a”. This si-
tuation triggers TR-WTAT (i.e. Condition in TR-
WTAT is satisfied). Thus, the Trigger Manager sends
a message to the General Manager containing the in-
struction to manage such an interaction (i.e. the Ma-
nag component in TR-WTAT, i.e. lines 2-6) and re-
moves TR-WTAT from the Interaction KB, since it is
not reusable during the patient treatment.
Then, the General Manager executes as a unique
transaction the instructions in the message, updating
the global vision. In our example, the instance of VT
in the yellow pages is updated by replacing WT with
the alternative plan (see lines 2-6 in TR-WTAT), as
shown in the lower part of Fig. 2. Thus, the General
Manager notifies to Executor
V T
that the instance of
VT in the yellow pages has been updated. As conse-
quence, Executor
V T
sends a message to Client
V T
to
refresh the visualization of VT, and let Physician
2
go
on with the execution of the updated CIG.
Moreover, let us suppose that, during the execu-
tion of such CIGs, the patient has a heart failure. As a
consequence TR-HF is triggered by the Trigger Ma-
nager. Then the Trigger Manager sends a message to
General Manager with the instructions to manage the
heart failure (lines 2-5 in TR-HF). The General Ma-
nager executes these instructions. The first two in-
structions (lines 2-3 in TR-HF) generate (both in the
graph of CIG dependencies and in the yellow pages
of CIGs) the node corresponding to the CIG to treat
heart failure. As a result of such a generation, our
framework supports the search for a physician accep-
ting the responsibility of executing the new CIG (fol-
lowing the approach in (Bottrighi et al., 2013)), and
generates a new instance of Executor module to ma-
nage the Heart Failure CIG. The selected physician
can manage the execution of the CIG trough a client.
In case s/he is already executing a CIG for the speci-
fic patient, the Heart Failure CIG is added to its client,
otherwise a new client is initialized for her/him. Mo-
reover, the interpretation of lines 4-5 in TR-HF adds
two (suspension) arcs in the graph of CIGs dependen-
cies, then the General Manager notifies the suspen-
sion to Executor
V T
and to Executor
PU
. Consequently,
the two executors notify the suspension to the corre-
sponding clients.
5 CONCLUSIONS
Traditional CIG execution engines provide physicians
with consolidated support for the execution of a sin-
gle CIG on a single patient. However, the treatment of
“exceptions” and of comorbidities demands for more
extended supports. Indeed, the management of such
phenomena requires also a support for the concurrent
execution of multiple CIGs on the same patient. The
approaches proposed in the literature manage either
“exceptions” or comorbidities, and do not provide fa-
cilities to cope with the coordination between such a
concurrent executions, which is an essential issue.
In this paper, we provide the first homogene-
ous framework for the management of both “excepti-
ons” and interactions dealing with the concurrent and
coordinate execution of multiple CIGs. Our appro-
ach is modular, in that it adds a further layer buil-
ding upon “traditional” execution engines for a sin-
HEALTHINF 2018 - 11th International Conference on Health Informatics
74

gle CIG. Though our framework is being built on top
of META-GLARE, our methodology is general, and
can be adapted for similar CIG systems (such as, e.g.,
PROforma (Fox et al., 1998) or Asbru (Shahar et al.,
1998)).
We are currently implementing our approach
using Java (Java-based prototypes of META-GLARE
and its extensions to cope with comorbid patients are
available). As soon as the implementation will be
completed, we plan to develop an extensive experi-
mentation of our framework, especially in the context
of comorbidity treatment. Moreover, we plan to ex-
tend our approach to provide a more comprehensive
support for distributed execution of CIGs to grant tre-
atment continuity, contextualization, and responsibi-
lity assignment and delegation.
ACKNOWLEDGMENTS
This research is original and has a financial support of
the Universit
`
a del Piemonte Orientale.
REFERENCES
Anselma, L., Bottrighi, A., Hommersom, A., Terenziani,
P., and Hunter, A. (2015). Supporting physicians
and patients through recommendation: Guidelines and
beyond. In Hommersom, A. and Lucas, P. J. F., edi-
tors, Foundations of Biomedical Knowledge Repre-
sentation - Methods and Applications, volume 9521 of
Lecture Notes in Computer Science, pages 281–286.
Springer.
Anselma, L., Piovesan, L., and Terenziani, P. (2017). Tem-
poral detection and analysis of guideline interactions.
Artificial Intelligence in Medicine, 76:40–62.
Barnett, K., Mercer, S. W., Norbury, M., Watt, G., Wyke,
S., and Guthrie, B. (2012). Epidemiology of multi-
morbidity and implications for health care, research,
and medical education: a cross-sectional study. The
Lancet, 380(9836):37–43.
Bottrighi, A., Chesani, F., Mello, P., Montali, M., Montani,
S., Storari, S., and Terenziani, P. (2009). Analysis
of the GLARE and GPROVE Approaches to Clinical
Guidelines. In KR4HC, volume 5943 of Lecture Notes
in Computer Science, pages 76–87. Springer.
Bottrighi, A., Leonardi, G., Piovesan, L., and Terenziani, P.
(2016). Knowledge-Based Support to the Treatment
of Exceptions in Computer Interpretable Clinical Gui-
delines:. International Journal of Knowledge-Based
Organizations, 6(3):1–27.
Bottrighi, A., Molino, G., Montani, S., Terenziani, P., and
Torchio, M. (2013). Supporting a distributed execu-
tion of clinical guidelines. Computer Methods and
Programs in Biomedicine, 112(1):200–210.
Bottrighi, A., Rubrichi, S., and Terenziani, P. (2015).
META-GLARE: A meta-engine for executing com-
puter interpretable guidelines. In Ria
˜
no, D., Lenz,
R., Miksch, S., Peleg, M., Reichert, M., and ten
Teije, A., editors, Knowledge Representation for He-
alth Care - AIME 2015 International Joint Workshop,
KR4HC/ProHealth 2015, Pavia, Italy, June 20, 2015,
Revised Selected Papers, volume 9485 of Lecture No-
tes in Computer Science, pages 37–50. Springer.
Bottrighi, A. and Terenziani, P. (2016). META-GLARE: A
meta-system for defining your own computer interpre-
table guideline system—Architecture and acquisition.
Artificial Intelligence in Medicine, 72:22–41.
Fox, J., Johns, N., and Rahmanzadeh, A. (1998). Dissemi-
nating medical knowledge: the PROforma approach.
Artificial Intelligence in Medicine, 14(1-2):157–181.
Fraccaro, P., Arguello Castelerio, M., Ainsworth, J., and
Buchan, I. (2015). Adoption of Clinical Decision Sup-
port in Multimorbidity: A Systematic Review. JMIR
Medical Informatics, 3(1):e4.
Grando, A., Peleg, M., and Glasspool, D. (2010). A goal-
oriented framework for specifying clinical guidelines
and handling medical errors. Journal of Biomedical
Informatics, 43(2):287–299.
Leonardi, G., Bottrighi, A., Galliani, G., Terenziani, P.,
Messina, A., and Corte, F. D. (2012). Exceptions
Handling within GLARE Clinical Guideline Frame-
work. In AMIA.
L
´
opez-Vallverd
´
u, J. A., Ria
˜
no, D., and Collado, A. (2013).
Rule-Based Combination of Comorbid Treatments for
Chronic Diseases Applied to Hypertension, Diabetes
Mellitus and Heart Failure. In Process Support and
Knowledge Representation in Health Care, volume
7738, pages 30–41. Springer Berlin Heidelberg, Ber-
lin, Heidelberg.
Merhej, E., Schockaert, S., McKelvey, T. G., and De Cock,
M. (2016). Generating conflict-free treatments for pa-
tients with comorbidity using ASP. In KR4HC 2016,
Lecture Notes in Computer Science, pages 93–100.
Michalowski, M., Wilk, S., Michalowski, W., Lin, D., Fa-
rion, K., and Mohapatra, S. (2013). Using Constraint
Logic Programming to Implement Iterative Actions
and Numerical Measures during Mitigation of Con-
currently Applied Clinical Practice Guidelines. In
Proceedings of AIME, number 7885 in Lecture Notes
in Computer Science, pages 17–22. Springer Berlin
Heidelberg.
Peleg, M. (2013). Computer-interpretable clinical guideli-
nes: A methodological review. Journal of Biomedical
Informatics, 46(4):744–763.
Peleg, M., Somekh, J., and Dori, D. (2009). A methodo-
logy for eliciting and modeling exceptions. Journal of
Biomedical Informatics, 42(4):736–747.
Peleg, M., Tu, S. W., Bury, J., Ciccarese, P., Fox, J.,
Greenes, R. A., Hall, R. W., Johnson, P. D., Jones,
N., Kumar, A., Miksch, S., Quaglini, S., Seyfang, A.,
Shortliffe, E. H., and Stefanelli, M. (2003). Com-
paring Computer-interpretable Guideline Models: A
Case-study Approach. JAMIA, 10(1):52–68.
Piovesan, L., Molino, G., and Terenziani, P. (2014). Sup-
porting Physicians in the Detection of the Interactions
A General Framework for the Distributed Management of Exceptions and Comorbidities
75

between Treatments of Co-Morbid Patients. In He-
althcare Informatics and Analytics: Emerging Issues
and Trends, pages 165–193. IGI Global.
Piovesan, L., Molino, G., and Terenziani, P. (2015). Suppor-
ting Multi-Level User-Driven Detection of Guideline
Interactions. In Proceedings of the International Con-
ference on Health Informatics (HEALTHINF-2015),
pages 413–422. Scitepress.
Piovesan, L. and Terenziani, P. (2015). A Mixed-Initiative
approach to the conciliation of Clinical Guidelines for
comorbid patients. In KR4HC 2015, volume 9485 of
Lecture Notes in Artificial Intelligence, pages 95–108.
Springer International Publishing, Pavia.
Quaglini, S., Stefanelli, M., Lanzola, G., Caporusso, V., and
Panzarasa, S. (2001). Flexible guideline-based patient
careflow systems. Artificial Intelligence in Medicine,
22(1):65–80.
Shahar, Y., Miksch, S., and Johnson, P. (1998). The Asgaard
project: a task-specific framework for the application
and critiquing of time-oriented clinical guidelines. Ar-
tificial Intelligence in Medicine, 14(1-2):29–51.
S
´
anchez-Garz
´
on, I., Fern
´
andez-Olivares, J., Onaindia, E.,
Milla, G., Jord
´
an, J., and Castej
´
on, P. (2013). A Multi-
agent Planning Approach for the Generation of Per-
sonalized Treatment Plans of Comorbid Patients. In
AIME 2013, pages 23–27.
Terenziani, P., Bottrighi, A., and Rubrichi, S. (2014).
META-GLARE: a meta-system for defining your
own CIG system: Architecture and Acquisition. In
KR4HC, pages 92–107.
Tu, S. W. and Musen, M. A. (1999). A flexible approach to
guideline modeling. Proceedings of the AMIA Sympo-
sium, pages 420–424.
van den Akker, M., Buntinx, F., Metsemakers, J. F., Roos,
S., and Knottnerus, J. A. (1998). Multimorbidity in
general practice: prevalence, incidence, and determi-
nants of co-occurring chronic and recurrent diseases.
Journal of Clinical Epidemiology, 51(5):367–375.
Wilk, S., Michalowski, M., Michalowski, W., Rosu, D.,
Carrier, M., and Kezadri-Hamiaz, M. (2017). Com-
prehensive mitigation framework for concurrent appli-
cation of multiple clinical practice guidelines. Journal
of Biomedical Informatics, 66:52–71.
HEALTHINF 2018 - 11th International Conference on Health Informatics
76
