Titanium Dioxide based Electrochromic Iris
Preparation, Characterization and Application
C. Kortz, A. Hein and E. Oesterschulze
Physics and Technology of Nanostructures, University of Kaiserslautern, Erwin-Schroedinger-Strasse 46,
67663 Kaiserslautern, Germany
Keywords:
Miniaturized Iris, Electrochromism, Viologen, Titanium Dioxide.
Abstract:
The miniaturization of a classical iris consisting of blades which are moved towards the path of light is limited,
due to the size of the actuators and the additional space needed for blade storage when the iris is completely
open. To overcome these limitations we present a fast switching, non-mechanical micro iris based on elec-
trochromic molecules, namely viologens, which are adsorbed onto a titanium dioxide nanoporous electrode.
Measurements of the energy consumption, the response time and the spectral light transmission are presented.
The complete fabrication route and the life time of the device are discussed in detail.
1 INTRODUCTION
The realization of a miniaturized iris comes to interest
since more and more commercially available hand-
held electronics were equipped with integrated cam-
eras. Due to the small size of these cameras a classical
blade iris cannot be integrated. An active iris is indis-
pensable for both the intensity and in particular the
depth of focus control.
a) b)
Figure 1: a) Images of a classical iris. In the upper left
corner the iris is completely open and only the blade storage
is left to be seen. This extra space is too big for micro iris
applications for integrated camera systems. b) A schematic
view of an electrochromic iris: the device can be switched
completely transparent, only a small area around the iris is
needed for sealing. This device can be miniaturized to fit
the conditions in integrated optics.
The major reason why there is no iris integrated
in small cameras is the required space. Especially
when the iris is completely open the blades have to be
moved outside the path of light and have to be stored
in an additional ring shaped space (see Fig. 1a)),
which is not available in micro optical devices.
Different solutions were presented to build up
miniaturised iris devices based on microelectrome-
chanical systems (MEMS), liquid displacement tech-
niques and electrochromic devices (Syms et al., 2004)
(Kimmle et al., 2011) (Deutschmann et al., 2015).
MEMS based iris devices have a similar working
principle as a classical blade iris (Yu et al., 2012). A
shutter is moved towards the path of light to realize
the iris function. The advantage of MEMS based de-
vices is the fast switching time. They can be miniatur-
ized easily but the dimensions of the device are much
larger than the path of light and high voltages of more
than 80 V are needed to move the blades. So they do
not fit the conditions for integrated optics in battery
powered hand-held electronics.
Another approach is to move light absorbing liq-
uids, like oil or ink into the path of light to create an
iris (Kimmle et al., 2011). The advantage is that in
this case the storage does not have to be ring shaped
around the optical path, but can be designed freely.
Nevertheless an additional space and actuators have
to be provided (M
¨
uller et al., 2012).
2 STATE OF THE ART
One approach to create a non-mechanical iris was us-
ing electrochromic molecules which change their ab-
sorption by applying a chemical potential (Roth et al.,
2011). Multiple designs and molecules were tested
(Deutschmann and Oesterschulze, 2014). The latest
iris was based on two complementary electrochromic
molecules, which were dissolved in the electrolyte en-
capsulated between two transparent conducting ox-
50
Kortz, C., Hein, A. and Oesterschulze, E.
Titanium Dioxide based Electrochromic Iris - Preparation, Characterization and Application.
DOI: 10.5220/0006552200500054
In Proceedings of the 6th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2018), pages 50-54
ISBN: 978-989-758-286-8
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
ide (TCO) coated glass sheets used as electrodes
(Deutschmann et al., 2015). If a voltage is applied
between these two electrodes the liquid turned from
transparent to almost black. This coloring process is
reversible, without any need for storage space. To
create the iris, the TCO layers were structured by
UV lithography. The dissolved electrochromophores
were only colored if the potential was applied at the
desired rings which form the iris shape (see Fig. 1b)).
A diffusional blurring of the iris structure was ob-
served which slightly influenced the iris function
(P
¨
atz et al., 2014).
The optical properties and the space and power
requirements of this non-mechanical iris were satis-
factory for integrated optics. But this design suffered
from long response times. Especially, the bleaching
took at least 20 s and longer. However, for consumer
electronics response times in a sub-second range are
required.
3 EXPERIMENTAL PROCEDURE
A transparent electrochromic iris device is realized
using two TCO coated glass substrates. These glasses
were cleaned in an ultrasonic bath using acetone, iso-
propanol and DI water for 5 min each and dried un-
der nitrogen flux. Alignment marks and contact pads
made of 10 nm Cr and 90 nm Au were added to the
TCO layer using magnetron sputtering and lift-off-
technique. In the next step, a paste containing tita-
nium dioxide (TiO
2
) nanoparticles with a diameter of
10-15 nm (Solaronix Ti-Nanoxide T/SP) was applied
by doctor-blading technique. To get the final porous
structure, the samples had to be heated up to 450
◦
C
on a hot plate and subsequently to 550
◦
C with a ramp
of 10
◦
C/min in a vacuum furnace. This temperature
was held for 2 h and the samples were cooled natu-
rally. The shape of the iris was created by laser struc-
turing. With this procedure the TiO
2
and the TCO
layers were structured in the same step. After this
preparation of the working electrode it was immersed
in a solution containing the viologen and kept there
for several hours.
The viologen we used in our experiments had a
phosphonate anchor group to ensure a surface occu-
pancy on the TiO
2
nanoporous layer, as seen in Fig. 2.
After the fictionalization was completed, the samples
were rinsed in ethanol and dried under nitrogen flux.
To build up a working device a second TCO coated
glass was bonded to the working electrode using a
UV-structurable spacer (Ordyl, Elga Europe). The
later carrying the cavity for the electrolyte. This cav-
ity could be filled and sealed hermetically to prevent
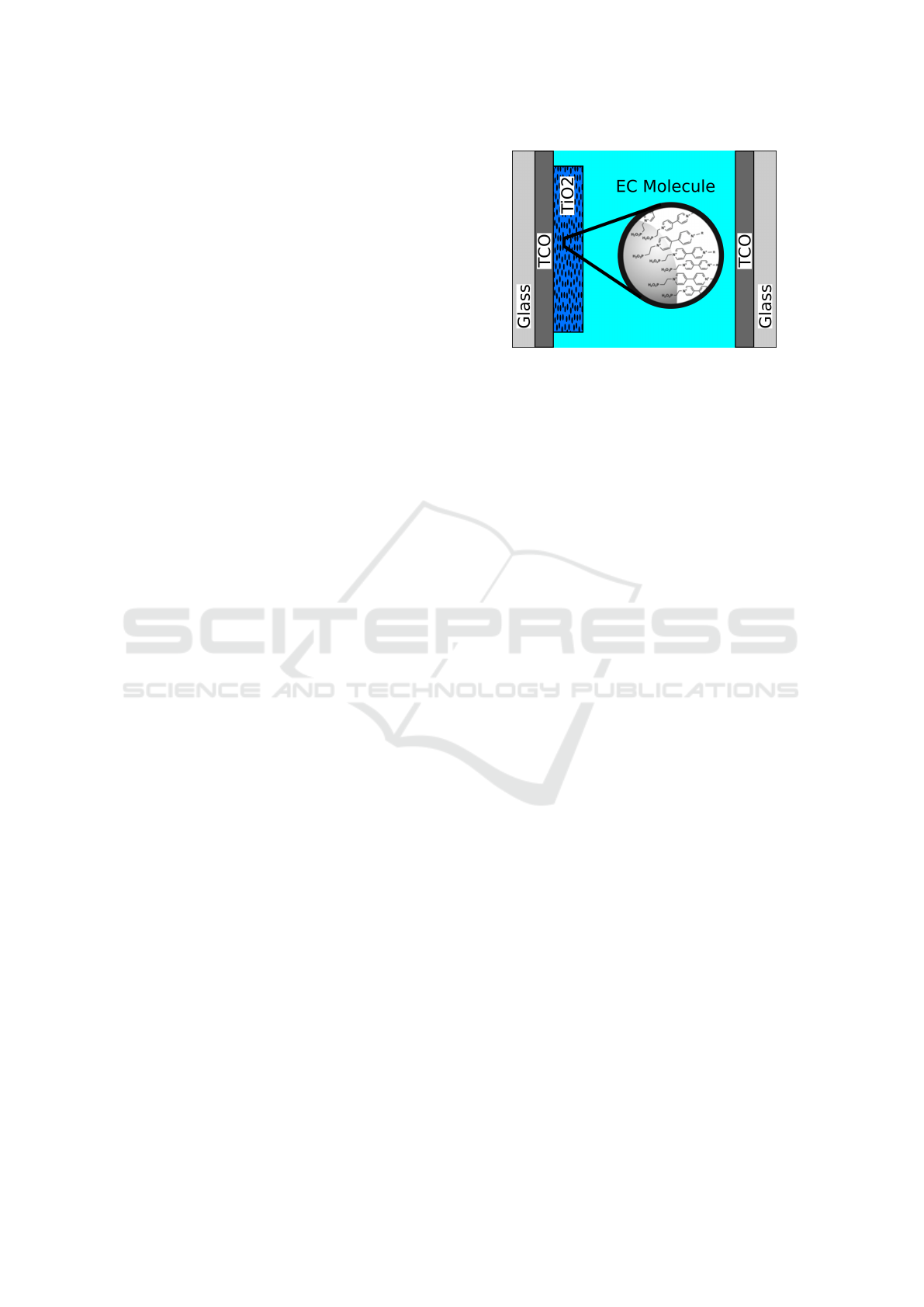
Figure 2: Schematic illustration of the electrochemical cell
device: the working electrode with nanoporous TiO
2
and
attached viologen on the left and the counter electrode on
the right side. The electrolyte in between provides charge
exchange among the electrodes.
oxygen and moisture infiltration.
Our goal is to realize an iris that fits all restric-
tions of miniaturized integrated camera systems for
consumer applications, which are:
• low space requirements;
• low power consumption;
• fast switching;
• high contrast;
• long lifetime.
These challenging restrictions are the major reasons
why integrated cameras are not equipped with an iris
yet.
4 RESULTS AND DISCUSSION
Each of the following sections addresses one of the
mentioned challenges. The newly developed solu-
tions, achievements and the current state of the re-
search are discussed in detail.
4.1 Structuring
The structuring of TiO
2
based electrochromic devices
is challenging, because the preferred UV lithography
techniques fail due to side reactions between the pho-
toresist and the TiO
2
nanoparticles during exposure.
For our device we chose to transfer the iris structure
into the nanoparticle layer by laser ablation (Roth,
2013).
With this method, the nanoparticle layer and the
TCO layer were structured simultaneously. As seen
in Fig. 3 the different iris rings are separated clearly
Titanium Dioxide based Electrochromic Iris - Preparation, Characterization and Application
51
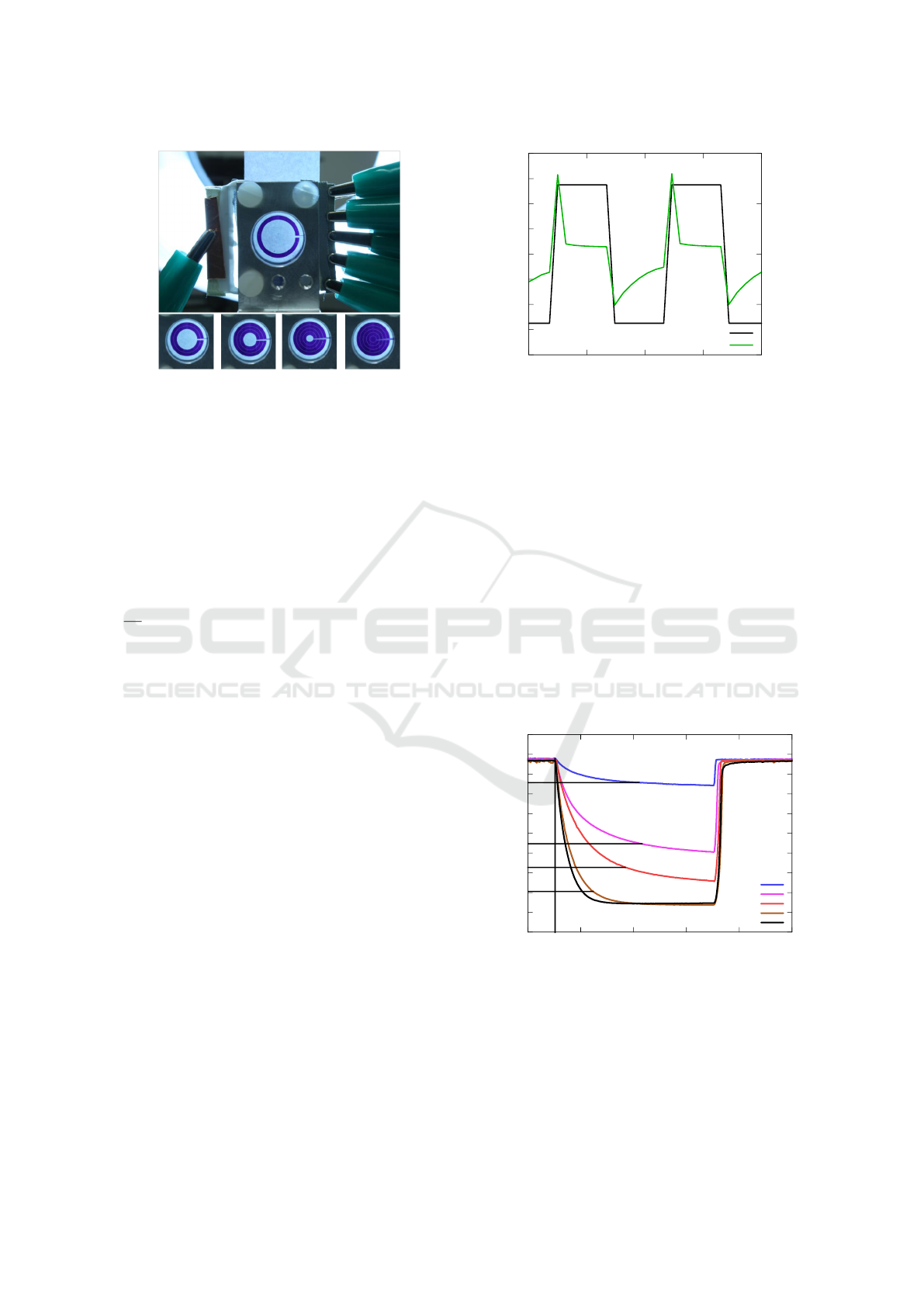
Figure 3: Images of a TiO
2
based iris in different switching
states (Roth, 2013).
from each other revealing a sharp edge. The conduc-
tor track to the contact pads on the right side interrupts
the outer rings.
4.2 Electrical Requirements
The electrical response was investigated operating the
working electrode in a cuvette using a commercially
available Ag/AgCl reference electrode and a Pt wire
as counter electrode. The electrolyte consisted of
1
mol
l
LiClO
4
salt solved in propylene carbonate. To
secure that no oxygen affects the measurement, the
electrolyte was bubbled for at least 15 min with nitro-
gen before use. The electric potential was applied and
the current was measured by a potentiostat (Refer-
ence600, Gamry Instruments). The potential between
the working electrode and the electrolyte leads to a
change in the redox state of the viologen. When a
negative potential is applied, the viologen molecules
are reduced and they change their color from trans-
parent to absorbing, resulting in a negative current.
Reversing the potential leads to the oxidation of the
viologen and they turn transparent again, resulting in
a positive current. This process is fast and reversible.
To keep the redox state no further current is needed; a
phenomenon that is called memory effect.
One of the restrictions for miniaturized iris de-
vices is low power consumption. As seen in Fig. 4
the maximum current needed to change the color of
the device was approximately 0.8 mA at a potential
of 0.55 V. This leads to a total maximum power of
0.44 mW, which can easily be provided by battery
power. As can be seen in Fig. 4 the current drops
rapidly after reaching its maximum value.
-0.8
-0.6
-0.4
-0.2
0
0.2
0.4
0.6
0.8
0 5 10 15 20
-1
-0.5
0
0.5
1
voltage [V]
current [mA]
time [s]
voltage against Ag/AgCl
current
Figure 4: Electrical response of the electrochromic working
electrode. A step potential was applied against a Ag/AgCl
reference electrode and the current was measured.
4.3 Electro-Chromophoric Response
The electro-chromophoric response was also mea-
sured in the previously described setup using a
Ag/AgCl reference electrode and a Pt counter elec-
trode in the same electrolyte. To observe the col-
oration, a homogeneous filter with an unstructured
working electrode was investigated. This filter was
illuminated by a white light source (KL 1500 LCD,
Schott) and the transmitted light was analyzed us-
ing an integrating sphere and a spectrometer (Flame,
Ocean Optics). To characterize the performance of
our device, the transmitted intensity was integrated
from 450 nm to 750 nm and recorded time-resolved.
0
10
20
30
40
50
60
70
80
90
100
0 10 20 30 40 50
transmission [%]
time [s]
-0.3 V
-0.35 V
-0.4 V
-0.5 V
-0.55 V
Figure 5: Time-resolved transmission through a homoge-
neous electro-chromic filter operated at different potentials.
Figure 5 shows the integrated intensity, where
100% stands for the light transmitted through the
measurement setup without the working electrode.
The maximum transmission through the working
electrode was 88% in the transparent state. The min-
imum transmission T
min
was 14% in the opaque state
PHOTOPTICS 2018 - 6th International Conference on Photonics, Optics and Laser Technology
52
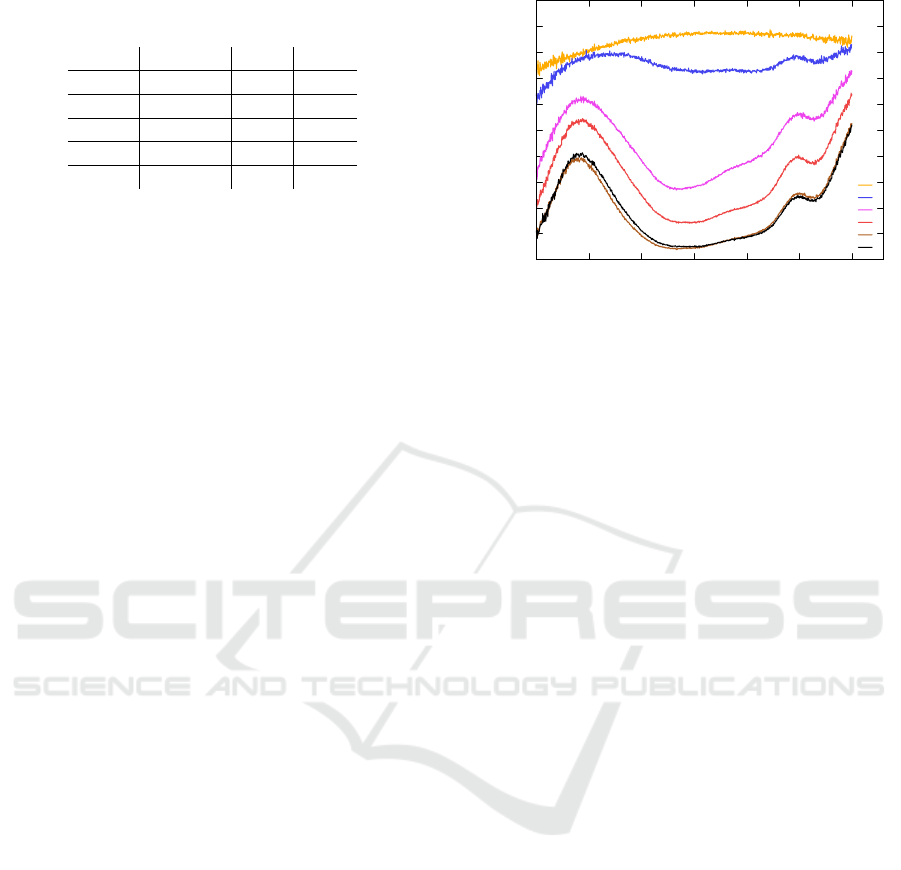
Table 1: Results taken from Fig. 5: minimum transmission
and response time at different applied potentials.
U [V] T
min
[%] t
c
[s] t
b
[s]
-0.3 73.8 16.0 0.2
-0.35 40.5 15.7 0.8
-0.4 25.5 13.4 1.2
-0.5 13.3 7.2 1.6
-0.55 13.7 5.2 1.5
at a potential of -0.55 V.
The response time was defined as the time where
90% of the equilibrium transmission is reached, indi-
cated by the horizontal lines in Fig. 5. We observed
a response time for coloration of t
c
= 5.0 s and for
bleaching of t
b
= 1.5 s when applying a potential of
±0.55 V.
The equilibrium transmission in the opaque state
can be controlled by the thickness of the TiO
2
layer,
the species and amount of EC-molecules adsorbed
onto the TiO
2
surface and the applied voltage. The
different T
min
responding to different applied poten-
tials are shown in Fig. 5, too. It is obvious that the
time for coloration is larger for low potentials al-
though the change in transmission is also low. The
resulting t
b
values do not strongly vary. The results
are summarized in Tab. 1.
The main reason why we do not reach 0% in
the colored state is the spectral characteristic of the
used viologen molecule. As can be seen in Fig. 6
the absorption deviates from the neutral behavior at
the wavelengths range from 450 nm to 750 nm. Es-
pecially around 500 nm and 750 nm large transmis-
sion in the colored state can be observed which is
characteristic for viologen molecules. This can af-
fect the function of the iris, especially the depth of
focus control, which was discussed in detail in (P
¨
atz
et al., 2014). The depth of focus differs compared to
a classical iris depending on the transmission through
the opaque region. Thus an effective aperture diame-
ter has to be defined to achieve comparable depth of
focus results.
In the transparent state we see a high transmission
which is constant over a large spectral range but drops
at 500 nm and shorter wavelengths. This behavior can
be explained by the absorption of the TCO, TiO
2
and
the viologen in its transparent state.
4.4 Hermetic Sealing
The device consists of two TCO coated glass sheets
with the mesoporous structure carrying the viologen
and the electrolyte in between. It was critical for the
electrolyte to be sealed leak proof and bubble free in
0
10
20
30
40
50
60
70
80
90
100
450 500 550 600 650 700 750
transmission [%]
wavelength [nm]
0 V
-0,3 V
-0,35 V
-0,4 V
-0,5 V
-0,55 V
Figure 6: Spectral transmission through a homogeneous vi-
ologen functionalized TiO
2
layer on a TCO substrate at dif-
ferent potentials.
the cavity between the glass sheets. But not only the
loss of the electrolyte destroys the function of the de-
vice, the penetration of oxygen and water affects the
switching, too. So even without a leakage, the func-
tionality of the device was reduced after days when
a non-hermetic sealant was used. So we included an
oxygen proof sealing made of UV curing adhesive.
This raised the durability of our cell from days to
months in first tests. Further investigations have to
be made in this field of interest to realize a durable
electrochromic device with the smallest possible di-
mensions.
5 CONCLUSION
A new approach to realize a non-mechanical miniatur-
ized iris based on viologen adsorbed onto nanoporous
TiO
2
was presented. The laser structuring of the
nanoporous TiO
2
layer showed satisfactory results
and the power consumption of the electrochromic
layer was suitable for battery powered devices. The
minimum transmission in the colored state reached
14% and could be varied by the applied potentials
which led to different response times of the elec-
trochromic layer. For long live stability a hermetical
sealing was used to ensure an oxygen and moisture
free electrolyte. This was realized by UV curing ad-
hesives.
ACKNOWLEDGEMENTS
We thank the German national research foundation
(DFG) for their financial support (project OE220/12-
2). We also acknowledge the nano structuring center
Titanium Dioxide based Electrochromic Iris - Preparation, Characterization and Application
53

(NSC) Kaiserslautern for their technical support and
Prof. Walder and Dr. Ciobanu for their assistance
and supply with the electrochromic molecules and the
electrolyte.
REFERENCES
Deutschmann, T., Kortz, C., Walder, L., and Oesterschulze,
E. (2015). High contrast electrochromic iris. Opt.
Express, 23(24):31544–31549.
Deutschmann, T. and Oesterschulze, E. (2014). Integrated
electrochromic iris device for low power and space-
limited applications. Journal of Optics, 16.
Kimmle, C., Schmittat, U., Doering, C., and Fouckhardt, H.
A. A. (2011). Compact dynamic microfluidic iris for
active optics. Microelectronic Engineering, 88:1772–
1774.
M
¨
uller, P., Feuerstein, R., and Zappe, H. A. (2012). In-
tegrated optofluidic iris. Journal of Microelectrome-
chanical Systems, 21:1156–1164.
P
¨
atz, D., Deutschmann, T., Oesterschulze, E., and
Sinzinger, S. (2014). Depth of focus analysis of opti-
cal systems using tunable aperture stops with a mod-
erate level of absorption. Applied optics.
Roth, S. (2013). Elektrochrome Absorber f
¨
ur die spektral-
laterale Filterung in der Mikrooptik. PhD thesis, Uni-
versity of Kaiserslautern.
Roth, S., Ignatowitz, M., M
¨
uller, P., M
¨
onch, W., and Oester-
schulze, E. (2011). Non-mechanical variable aper-
tures based on poly(3,4-ethylenedioxythiophene) (pe-
dot). Microelectronic Engineering, 88:2349–2351.
Syms, R. R. A., Zou, H., Stagg, J., and Veladi, H. (2004).
Sliding-blade mems iris and variable optical attenua-
tor. Journal of Micromechanics and Microengineer-
ing, 14(12):1700.
Yu, H., Guangya, Z., Du, Y., Mu, X., and Chau, F. S. A.
(2012). Mems-based tunable iris diaphragm. Journal
of microelectromechanical systems, 21:1136–1145.
PHOTOPTICS 2018 - 6th International Conference on Photonics, Optics and Laser Technology
54
