
An Investigation of How Wavelet Transform Can Affect the Correlation
Performance of Biomedical Signals
The Correlation of EEG and HRV Frequency Bands in the Frontal
Lobe of the Brain
Ronakben Bhavsar, Neil Daveya and Yi Sun and Na Helian
The School of Computer Science, University of Hertfordshire, Hatfield, U.K.
Keywords:
EEG, HRV, Biomedical Signal Processing, Time series Data Analysis, Pearson Correlation, Wavelet Trans-
form, Independent Component Analysis, Feature Extraction, Fast Fourier Transform.
Abstract:
Recently, the correlation between biomedical signals, such as electroencephalograms (EEG) and electrocar-
diograms (ECG) time series signals, has been analysed using the Pearson Correlation method. Although
Wavelet Transformations (WT) have been performed on time series data including EEG and ECG signals, so
far the correlation between WT signals has not been analysed. This research shows the correlation between
the EEG and HRV, with and without WT signals. Our results suggest electrical activity in the frontal lobe of
the brain is best correlated with the HRV. We assume this is because the frontal lobe is related to higher mental
functions of the cerebral cortex and responsible for muscle movements of the body. Our results indicate a
positive correlation between Delta, Alpha and Beta frequencies of EEG at both low frequency (LF) and high
frequency (HF) of HRV. This finding is independent of both participants and brain hemisphere.
1 INTRODUCTION
Biomedical signals are a record of electrical activity
within human body, and they may indicate the state
of health of human. Among many biomedical sig-
nals, Electroencephalograph (EEG) and Electrocar-
diograph (ECG) signals are considered in this work.
EEG signals provide a measure of brain nerve cell
electro-physiological activity, that is accessible on the
surface of the scalp (Lewis et al., 1988), thus pro-
vide information about different types of brain activ-
ity. Identifying changes in EEG signals has improved
our understanding of the relationship of these signals
to people
0
s moods, and behaviour (Han et al., 2012),
(Ebersole and Pedley, 2003). ECG signals contains
a plethora of information on the normal and patho-
logical physiology of the heart and its health. Fur-
thermore, ECG signals provide vital information with
regards to the function and rhythm of the heart. The
heart rate variability (HRV) has been extracted from
the ECG signals. HRV describes the variation in time
between consecutive heart beats, which is commonly
referred to as the RR (R wave to R wave) or NN (Nor-
mal beat to normal beat) intervals.
In recent years, the correlation between the EEG
and the ECG have been conducted to analyse their
functionality under certain conditions and to check
whether this functionality is related to each other. Re-
search (Kim et al., 2013), (Chua et al., 2012), (Ab-
dullah et al., 2009), (Sakai et al., 2007), (Berg et al.,
2005), (Edlinger and Guger, 2006), suggests that the
correlation between spectral bands of EEG and HRV
has been conducted to assess the interaction between
them, and achieved remarkable correlation.
The recent research on correlation between these
two signals as mentioned earlier has focused on the
Fourier analysis of the frequencies presents in these
signals. Whilst, the wavelet transform (WT), acts on
frequency and time of the recorded signals. There-
fore, WT has widely utilized for analysing biomedi-
cal or time series signals. The WT of the signal can
be thought of as an extension of the classic Fourier
transform (FT) - it works on multi-scale basis, instead
of working on a single scale (Time or Frequency) as
FT, and gives detailed and clear information of the
signals. Therefore, WT of the signals is an important
method not only to analyse EEG and ECG/HRV sig-
nals individually, but also to analyse the correlation
between them. According to recent research (Thomas
and Moni, 2016), (Chandra et al., 2017), (Mirsadeghi
Bhavsar, R., Davey, N., Sun, Y. and Helian, N.
An Investigation of How Wavelet Transform Can Affect the Correlation Performance of Biomedical Signals - The Correlation of EEG and HRV Frequency Bands in the Frontal Lobe of the
Brain.
DOI: 10.5220/0006551001390146
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 4: BIOSIGNALS, pages 139-146
ISBN: 978-989-758-279-0
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All r ights reserved
139

et al., 2016), (Mporas et al., 2015), (Valderrama et al.,
2012), (Nasehi and Pourghassem, 2011), (Cvetkovic
et al., 2008), WT has been used to analyse either
EEG or ECG signal, but the correlation between these
transformed signals has not yet been conducted. In
this paper we are not only focusing on the correlation
between without wavelet transform signals but also
between wavelet transformed signals.
2 RELATED WORK
A series of data points in time order, or time series,
provide the view of a signal as it evolves over time, in
the Time domain (TD). TD analysis is used to anal-
yse the signal in its actual state - it is utilised to anal-
yse changes in biomedical signals, such as the power
(or amplitude) over time. In addition, the frequencies
present in the signal are open to investigation (for ex-
ample, by using the Fast Fourier Transform (FFT)).
Such an analysis is said to take place in the Frequency
domain (FD). The FD analysis is used to identify fre-
quencies present in the signals. Furthermore, it can be
utilized to establish the relationship between frequen-
cies and its corresponding power (amplitude), and so
the energy distributions in signals.
In recent research, the correlation between EEG
and ECG/HRV signals have been analysed in the FD
,as shown in Table 1, which indicates that the Pear-
son correlation is the best method for the FD analy-
sis. In addition, different numbers of EEG electrodes
have been used to analyse the relationship with the
ECG/HRV. To the best of our knowledge, very lim-
ited work has been done on the correlation between
EEG and ECG/HRV signals using 19 EEG electrodes.
Moreover, no one has analysed these signals under the
same condition (i.e. with TEAS acupuncture applied)
that utilised in this research. This paper investigates
the correlation between EEG and ECG/HRV signals
in FD using Pearson correlation considering all 19
EEG electrodes under the same condition.
Based on the research as shown in Table 2 on WT,
it is straightforward that the DWT based methods are
well known for EEG and ECG feature extraction and
analysis. Furthermore. Among the DWT based meth-
ods mentioned, db wavelet method has been consid-
ered by the researchers. It is obvious from the re-
search on WT that key features of EEG and ECG sig-
nal can improve the analysis performance. Therefore,
it is important to analyse not just either EEG or ECG
as shown in Table 2, but also the correlation between
EEG and ECG. To our knowledge, we have not yet
found information on the correlation between wavelet
transformed signals. In this work, we describes such
an analysis.
3 DATASET INFORMATION
Two different datasets were obtained with each of
them containing different numbers of participants,
stimulation location, and total time length as shown in
Table 3. All of these datasets follow the 10-20 elec-
trode placement system shown in Figure 1. The 10-20
system is the recognized method to describe the lo-
cation of electrodes (Klem et al., 1999). The values
of 10 and 20 percentage shown in Figure 1 refer to
the distances between adjacent electrodes: either 10
or 20 percentage of the total front-to-back or right-to-
left distance over the skull - front-to-back distance is
based on the measurement from the Nasion (point be-
tween forehead and nose) to the Inion (lowest point
of the skull from the back of the head indicated by a
prominent bump), and right-to-left distance is based
on the measurement between the left and right preau-
ricular ear points.
Dataset 1 and 2 consist of EEG and ECG recordings
from 16 and 7 participants, respectively. These data
were obtained over ten 5 minutes slots with eyes open
using Transcutaneous Electro Acupuncture (TEAS)
method, including resting state data in the first and
the last slot. The EEG and ECG recording were made
simultaneously. 19 electrodes (Fp1, Fp2, F7, F3, Fz,
F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1,
and O2) for EEG recording were used, following the
10-20 system. The sampling rate used for EEG was
250Hz, and the reference was to linked ear electrodes.
For ECG data, two electrodes were placed on both
side of the wrist (having one electrode as ground) to
record the electrical activity of the heart over time,
and the sampling rate used was 256Hz.
Table 3: Information about the Datasets.
Label Dataset 1 Dataset 2
Number of Participants 16 7
EEG-Electrodes 19 19
EEG-Sampling Rate 250Hz 250Hz
ECG-Electrode 1 1
Stimulation Location 1 4
ECG-Sampling Rate 256Hz 256Hz
Total Time Length 50 minutes 45 minutes
Slot Time Length 5 minute 5 minute
The difference between these datasets, other than
the participants, is the body location where TEAS
stimulation has been performed. For Dataset 1, only
one body location (Dominant Hand), and for Dataset
2, four different body location (Left Hand, Below Left
Knee, Right Hand, and Below Right Knee) has been
used to perform TEAS stimulation.
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
140
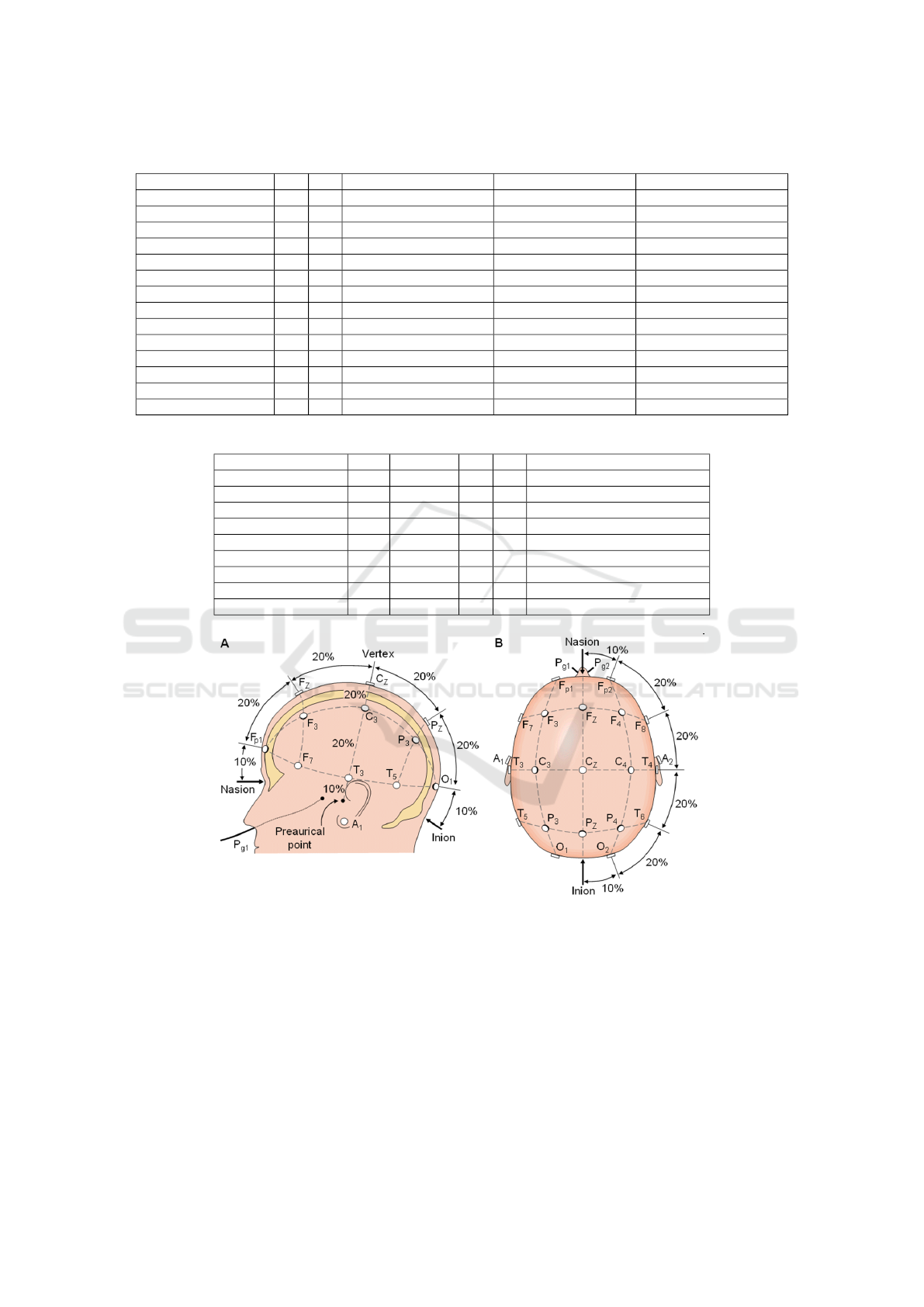
Table 1: Summary of Correlation Research on Biomedical Signals since 2003 to 2017.
RefDetail TD FD Pearson Correlation Method Other Correlation Method EEG Electrodes Investigated
(Miyashita et al., 2003) - X X - 4
(Yang et al., 2002) - X X - 2
(Ako et al., 2003) - X X - 1
(Jurysta et al., 2003) - X - Coherency Analysis 3
(Takahashi et al., 2005) - X X - 6
(Edlinger and Guger, 2006) - X X - 2
(Berg et al., 2005) - X X - 2
(Sakai et al., 2007) - X X - 19
(Abdullah et al., 2010) - X - Cross-correlation 1
(Chua et al., 2012) - X - X 4
(Kim et al., 2013) - X - Coherency Analysis 19
(Prinsloo et al., 2013) X - X - 3
(Liou et al., 2014) - X X - 19
(Triggiani et al., 2016) - X X - 19
Table 2: Summary of Research on Well known Wavelet Transformation Methods for Biomedical Signals since 2012 to 2017.
RefDetail EEG ECG/HRV TD FD Feature Extraction Method
(Kutlu and Kuntalp, 2012) - X X - DWT-Daub Wavelet
(Thomas et al., 2015) - X X - DWT-Daub Wavelet
(Sudarshan et al., 2017) - X X - DWT-Daub Wavelet
(Acharya et al., 2017) - X - X DWT-Daub Wavelet
(Dolatabadi et al., 2017) - X X X Principal Component Analysis (PCA)
(Kumari et al., 2014) X - X X DWT-Daub Wavelet
(Mumtaz et al., 2017) X - X X DWT-Daub Wavelet
(Kevric and Subasi, 2017) X - - X DWT-Daub Wavelet
(Faust et al., 2015) X - X - DWT-Daub Wavelet
Figure 1: The international 10-20 system seen from A (left side of the head) and B (above the head). The letter F, T, C, P, O,
A, Fp and Pg stands for frontal, temporal, central, parietal, occipital, earlobes, frontal polar, and nasopharyngeal, respectively.
The figure is obtained from (Klem et al., 1999).
4 METHODS
4.1 Pearson Correlation
The Pearson
0
s correlation coefficient measures how
closely two different observables are related to each
other. Correlation co-efficient range between 1 (when
the matching entities are exactly the same) and −1
(when the matching entities are inverses of each
other). A value of zero indicates no relationship ex-
isting between the entities.
4.2 Wavelet Transform
The Wavelet Transform (WT) is designed to direct
the problem of signals with nonstationarity. It in-
An Investigation of How Wavelet Transform Can Affect the Correlation Performance of Biomedical Signals - The Correlation of EEG and
HRV Frequency Bands in the Frontal Lobe of the Brain
141
cludes representation of time function in terms of sim-
ple blocks, termed wavelets. These blocks are derived
from a signal generating function called the mother
wavelet by translation and dilation operations. Dila-
tion, also known as scaling, compresses or stretches
the mother wavelet and translation shifts it along the
time axis (Daubechies, 1990), (Akay, 1997), (Unser
and Aldroubi, 1996). The WT can be categorized into
continuous and discrete. Continuous wavelet trans-
form (CWT), implies that the scaling and translation
parameters change continuously, and thus, represent
considerable effort and vast amount of data calcula-
tion for every possible scale. Therefore, we used dis-
crete wavelet transform (DWT). The WT of the sig-
nal can be thought of as an extension of the classic
Fourier transform (FT) - it works on multi-scale ba-
sis, instead of working on a single scale (Time or Fre-
quency) as FT. This is achieved by decomposition of
the signal over dilated (scale) and translated (time)
version of wavelet. An input signal is decomposed by
using low pass filter and high pass filter followed by
down sampling in each stage. The output of the first
stage high pass filter gives the detail coefficient (D1),
whereas the low pass filter gives the approximation
coefficient (A1).
The prototype wavelet used in this study is
Daubechies wavelet of order 4 (db4) based on our re-
search on biomedical/time series signal analysis, as
mentioned in Table 2.
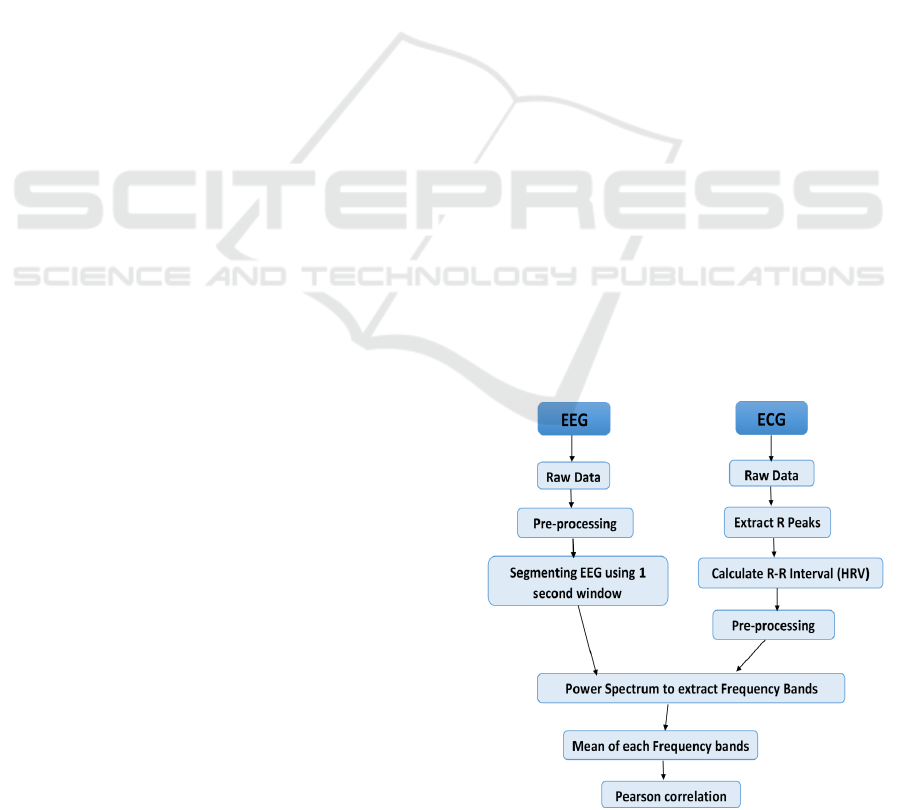
5 EXPERIMENTAL SET-UP
The experimental steps are shown in Figure 2. The
EEG signals were pre-processed to remove artefacts
caused by the electrical activity in muscles includ-
ing eye, jaw and muscle movements using Indepen-
dent Component Analysis (ICA). It was straightfor-
ward to remove these using ICA (Hyv
¨
arinen and Oja,
2000). The power spectrum for each frequency band
of EEG - Delta (0.3-4 Hz), Theta (4-7.5 Hz), Alpha
(7.5-13 Hz), Beta (13-30 Hz), and Gamma (30-50
Hz) were then obtained by Power Spectrum Density
(PSD) (Stoica and Moses, 1997).
To extract HRV from ECG signals, we used
method designed by Lin et al. (Lin et al., 2010).
The results of the automatic analysis were reviewed
and any errors in R-wave detection and QRS labelling
were then removed manually. R-R interval data ob-
tained from the edited time sequence of R-wave and
QRS labelling were then transferred to a personal
computer. In order to remove artefact from extracted
HRV signal, each R-R interval has been compared
against a local average interval. If an R-R interval
differs from the local average more than a specified
threshold (Threshold in seconds) value, then that R-
R interval is defined as an artefact and is replaced
with an interpolated value using a cubic spline in-
terpolation. The power spectrum for each frequency
band of HRV - Very Low Frequency (VLF) ranges 0-
0.04 Hz, Low Frequency (LF) ranges 0.04-0.15 Hz,
and High Frequency 0.15-4 Hz were then obtained by
PSD (Power Spectrum Density).
The sampling rate is 1Hz for the extracted HRV,
and 250Hz for the EEG. In order to perform corre-
lation between these different sampling rate signals,
it was required to change the sampling rate for either
the EEG or HRV signals. Therefore, we decided to
segmenting EEG signals using 1 second window and
represent each window by its means value (the mean
value from each 250 samples), unlike normal down
sampling, where much of the data is thrown away.
For each participant’s EEG data, this process has been
repeated for all 5 minutes slots. After windowing,
the spectral analysis was performed. From each fre-
quency bands of the EEG and the HRV, the mean of
the amplitude value within the frequency range has
been measured, single value for each of these fre-
quency band, and for each 5 minute is obtained. Then,
the correlation between these frequency values is per-
formed.
In order to perform correlation based on wavelet
transformed EEG and/or HRV signal, the WT-
Daubechies (db) Wavelet up to level 5 is performed
on the signals before extracting frequency bands as
mentioned in Figure 2. For the datasets we have, the
low pass filter worked very well. Therefore, we con-
sidered low passed WT signals to perform the corre-
lation.
Figure 2: Experiment steps for the correlation performance.
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
142

6 EXPERIMENTAL RESULTS
AND DISCUSSION
For each dataset, we investigated the correlation be-
tween each of the EEG frequencies (Delta, Theta, Al-
pha and Beta) with each frequencies of the HRV fre-
quencies (LF and HF) in three different experiments:
1). The Correlation between Pre-processed Signals,
2). The Correlation between Pre-processed and WT
signals of the EEG and HRV, and finally 3). The
Correlation between Pre-processed HRV with Pre-
processed and WT signals of the EEG. The Gamma
frequency of EEG did not give us the correlation ef-
fect. Therefore, it is not considered in the result
shown in Figure 3 and Table 4.
For both datasets, the experiment 2). correlation
between both WT signals did not give better results,
because HRV is tend to be less noisy. Therefore, when
the WT has been performed on HRV, information has
been lost and the signal became more flat. The most
interesting result has been found from experiments 1).
and 3).
For each frequency combination correlation, the
average of participants for each EEG electrode has
been calculated. Then the best performance electrode
has been ranked- where, the ranking has been given
based on electrode correlation result. The average of
electrode ranking for each frequency combination is
then gathered and five best performance electrodes re-
sult has been looked closely. We have found some
common electrodes in all of the frequency combina-
tion we have investigated. Figure 3 shows the result
of this investigation for Dataset 1 and 2.
As shown in Figure 3, for dataset 2, some elec-
trodes from the back side of the brain are giving
stronger result than dataset 1. This is due to more
randomness in the EEG signals from dataset 2. Also,
the location where TEAS has been performed might
contributed to this result.
Based on results shown in Figure 3, it can be
seen that the frontal lobe of the brain is correlated
with the heart. The frontal lobe involved in higher
mental functions, such as concentration, creativity,
speaking, muscle movement and in making plans and
judgements, is a part of cerebral cortex (body’s ulti-
mate control and information processing) of the brain
(McCraty et al., 2009). The usual Heart-Brain com-
munication path is through spinal cord. In order to
have relationship between frontal lobe of the brain
and heart, we assume the communication might have
done through ’Medulla’(cardiovascular center placed
in medullacontrols the heart beating) which is part of
brain stem. The signal has been then directed to the
Thalamus and then to the cerebral cortex (Lane et al.,
2001), (ATKINSON and BRADLEY, 2004).
Table 4 shows the average correlation result of
participants for each frequency comparison from
dataset 1 and 2. Where, Level 0 means correla-
tion between pre-processed data, and Level 1 to 5
means, correlation between pre-processed HRV with
pre-processed and WT EEG. The heat map of these
result (”Red” is strongest and ”Dark-Blue” means
weakest) as shown in Table 4, indicates the correla-
tion performance changes with the levels of WT. We
found the signal became flat after level 2, and lost in-
formation when levels has been increased. Therefore,
we have not considered result of levels 3, 4 and 5 in
Figure 3 (b) and (d).
Results shown in Table 4 are indicative and not
statistically significant, according to these, three fre-
quencies of EEG have shown some correlation, such
as Delta, Alpha, and Beta, have shown correlation at
both LF and HF of HRV. Each of these frequencies
represent the activities of these signals. For example,
Delta will be higher if the person is in deep sleep, Al-
pha will appear if the person is calmed, relaxed or
in creative visualisation, and Beta will show if the
person is working or feeling more alert. For HRV,
LF and HF represent the sympathetic and parasympa-
thetic activities of autonomic nervous system (ANS),
respectively.
7 CONCLUSIONS
The main conclusion of this work is that electrical ac-
tivity in the frontal lobe of the brain is correlated with
the HRV for the given two datasets. To the best of
our knowledge this is a new result. This suggests that
most probably the electrical signals could be trans-
mitted through the cerebral cortex, Thalamus, and
Medulla of the brain (Saper et al., 2005). The possi-
ble path of the key neuronal projections that maintain
alertness is shown in Figure 4.
The second conclusion from this work is that, WT
signals also give correlation from the frontal lobe of
the brain. To the best of our knowledge, the correla-
tion between WT signals of EEG and ECG/HRV has
not yet been investigated.
A more tentative conclusion of this work is that
three frequencies of the EEG Delta, Alpha and Beta
are correlated with the LF and HF of HRV, for dataset
1 and dataset 2, respectively. Whereas, most of previ-
ous studies, (Yang et al., 2002),(Ako et al., 2003),(Ju-
rysta et al., 2003),(Abdullah et al., 2010) and (Chua
et al., 2012), have shown negative correlation between
these frequency bands due to the condition in which
these signals have been analysed.
An Investigation of How Wavelet Transform Can Affect the Correlation Performance of Biomedical Signals - The Correlation of EEG and
HRV Frequency Bands in the Frontal Lobe of the Brain
143
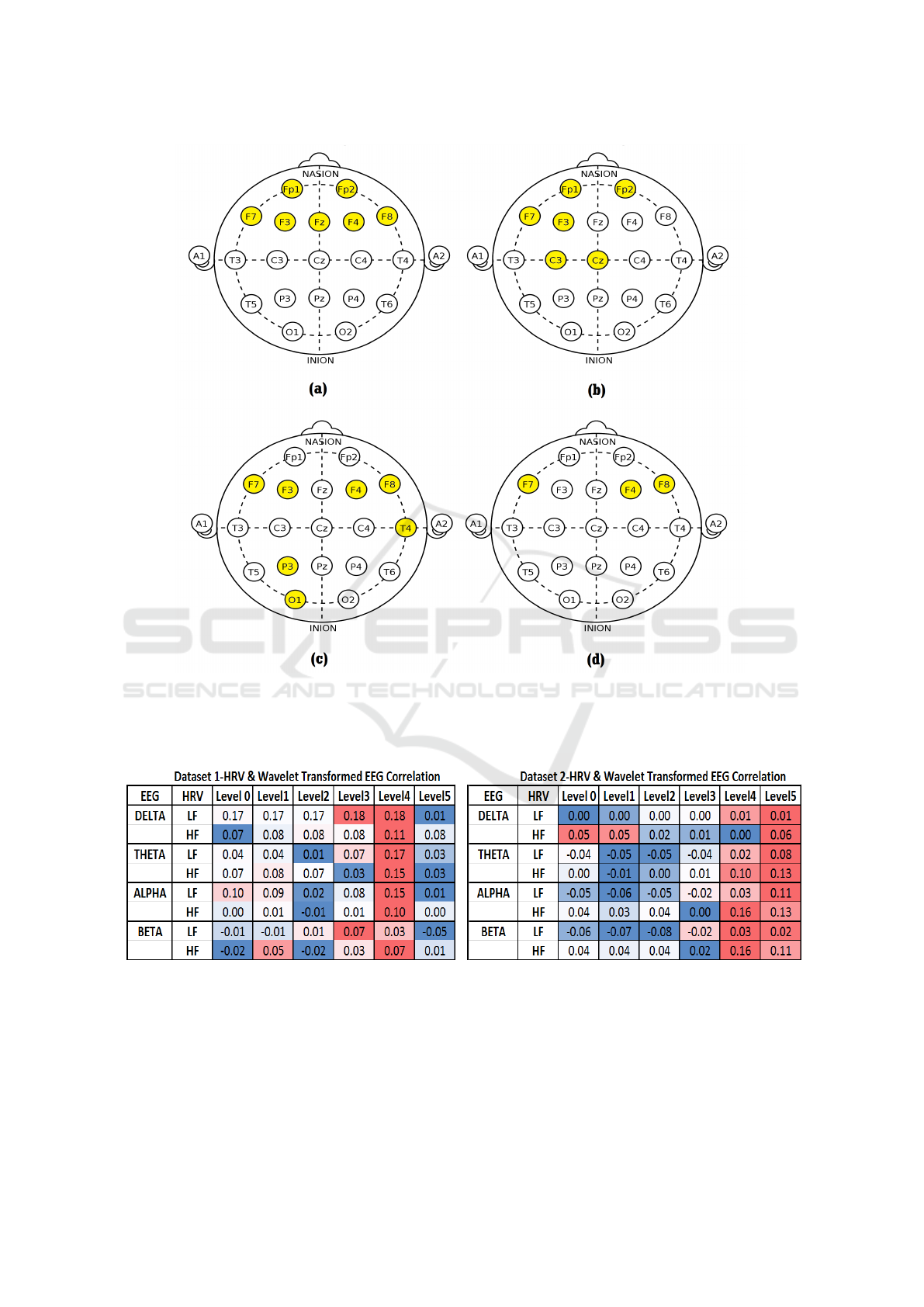
Figure 3: Best Electrodes Correlation Performance, highlighted in yellow colour: (a) Dataset 1 Correlation performance
on pre-processed HRV and EEG, (b) Dataset 1 Correlation performance on pre-processed HRV and WT signals of EEG,(c)
Dataset 2 Correlation performance on pre-processed HRV and EEG, (d) Dataset 2 Correlation performance on pre-processed
HRV and WT signals of EEG.
Table 4: Heat Map Results of Averaged participants correlation performance: Dataset 1 (Left), and Dataset 2 (on Right).
Colour coding from Red to Dark Blue, Red=Strongest, Dark-Blue=Weakest).
In summary, the number of EEG electrodes used
by other people to investigate correlation was limited.
Our results cover a gap in the research concerning the
correlation between the EEG and the HRV using all
EEG electrodes. Our work suggests a correlation be-
tween the frontal lobe of the EEG and the HRV, with
and without WT signals. We assume this is because
the frontal lobe is related with higher mental functions
of cerebral cortex and responsible for muscle move-
ments of the body (Stuss and Benson, 1986).
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
144
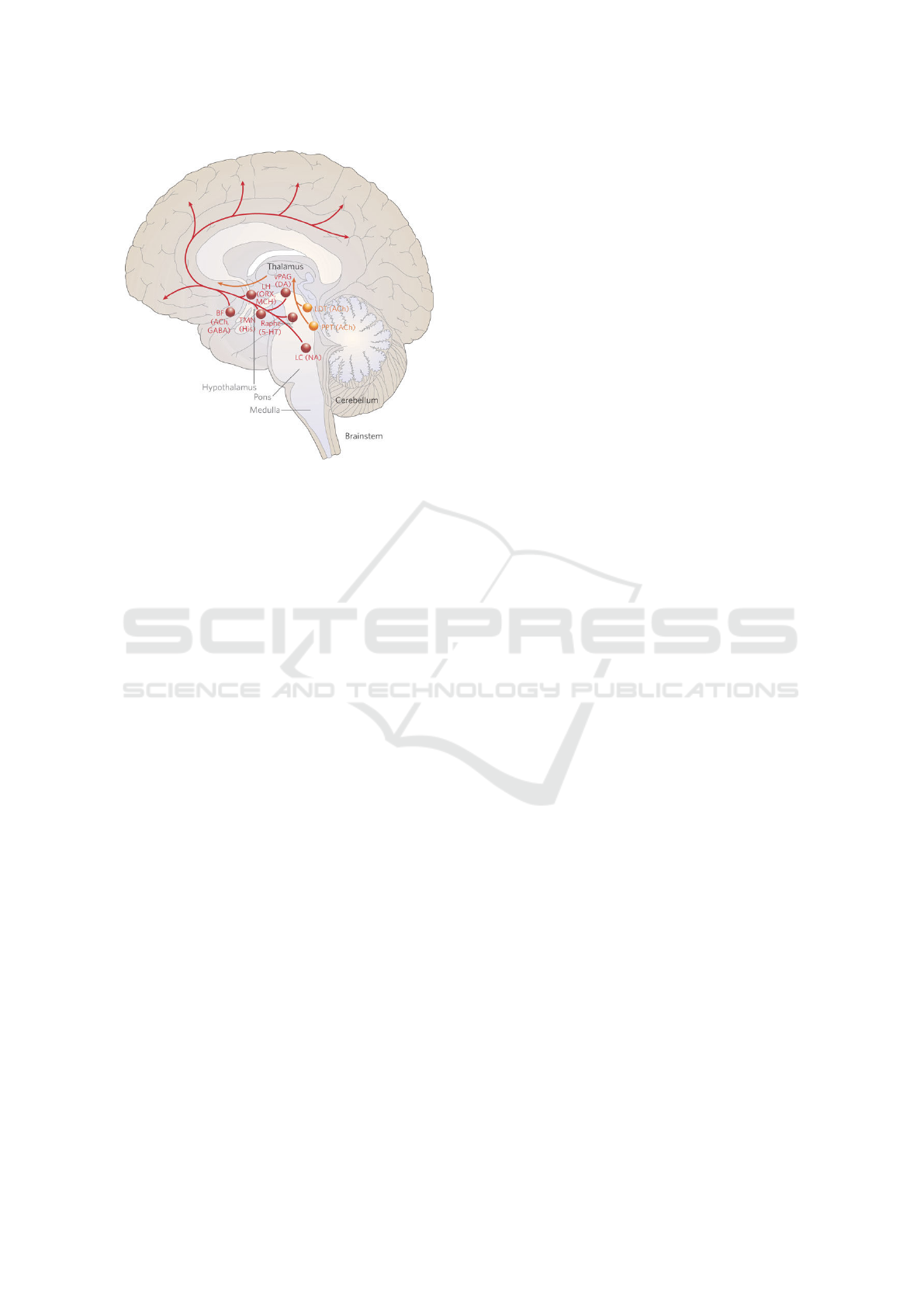
Figure 4: Key neuronal projections that maintain alertness,
and possibly the path from cardiovascular center to the
frontal lobe of the barin’s communication. The figure is
obtained from (Saper et al., 2005).
REFERENCES
Abdullah, H., Holland, G., Cosic, I., and Cvetkovic, D.
(2009). Correlation of sleep eeg frequency bands
and heart rate variability. In Engineering in Medicine
and Biology Society, 2009. EMBC 2009. Annual Inter-
national Conference of the IEEE, pages 5014–5017.
IEEE.
Abdullah, H., Maddage, N. C., Cosic, I., and Cvetkovic,
D. (2010). Cross-correlation of eeg frequency bands
and heart rate variability for sleep apnoea classifica-
tion. Medical & biological engineering & computing,
48(12):1261–1269.
Acharya, U. R., Fujita, H., Adam, M., Lih, O. S., Sudar-
shan, V. K., Hong, T. J., Koh, J. E., Hagiwara, Y.,
Chua, C. K., Poo, C. K., et al. (2017). Automated
characterization and classification of coronary artery
disease and myocardial infarction by decomposition
of ecg signals: a comparative study. Information Sci-
ences, 377:17–29.
Akay, M. (1997). Wavelet applications in medicine. IEEE
spectrum, 34(5):50–56.
Ako, M., Kawara, T., Uchida, S., Miyazaki, S., Nishihara,
K., Mukai, J., Hirao, K., Ako, J., and Okubo, Y.
(2003). Correlation between electroencephalography
and heart rate variability during sleep. Psychiatry and
clinical neurosciences, 57(1):59–65.
ATKINSON, M. and BRADLEY, R. T. (2004). Electro-
physiological evidence of intuition: Part 2. a system-
wide process? THE JOURNAL OF ALTERNATIVE
AND COMPLEMENTARY MEDICINE, 10(2):325–
336.
Berg, J., Neely, G., Wiklund, U., and Landstr
¨
om, U. (2005).
Heart rate variability during sedentary work and sleep
in normal and sleep-deprived states. Clinical physiol-
ogy and functional imaging, 25(1):51–57.
Chandra, S., Sharma, G., Sharma, M., Jha, D., and Mit-
tal, A. P. (2017). Workload regulation by sudarshan
kriya: an eeg and ecg perspective. Brain informatics,
4(1):13.
Chua, E. C.-P., Tan, W.-Q., Yeo, S.-C., Lau, P., Lee,
I., Mien, I. H., Puvanendran, K., and Gooley, J. J.
(2012). Heart rate variability can be used to estimate
sleepiness-related decrements in psychomotor vigi-
lance during total sleep deprivation. Sleep, 35(3):325–
334.
Cvetkovic, D.,
¨
Ubeyli, E. D., and Cosic, I. (2008). Wavelet
transform feature extraction from human ppg, ecg,
and eeg signal responses to elf pemf exposures: A pi-
lot study. Digital signal processing, 18(5):861–874.
Daubechies, I. (1990). The wavelet transform, time-
frequency localization and signal analysis. IEEE
transactions on information theory, 36(5):961–1005.
Dolatabadi, A. D., Khadem, S. E. Z., and Asl, B. M. (2017).
Automated diagnosis of coronary artery disease (cad)
patients using optimized svm. Computer methods and
programs in biomedicine, 138:117–126.
Ebersole, J. S. and Pedley, T. A. (2003). Current practice of
clinical electroencephalography. Lippincott Williams
& Wilkins.
Edlinger, G. and Guger, C. (2006). Correlation changes of
eeg and ecg after fast cable car ascents. In Engineering
in Medicine and Biology Society, 2005. IEEE-EMBS
2005. 27th Annual International Conference of the,
pages 5540–5543. IEEE.
Faust, O., Acharya, U. R., Adeli, H., and Adeli, A. (2015).
Wavelet-based eeg processing for computer-aided
seizure detection and epilepsy diagnosis. Seizure,
26:56–64.
Han, M., Sun, L., and Hong, X. (2012). Extraction of the
eeg signal feature based on echo state networks. Sheng
wu yi xue gong cheng xue za zhi= Journal of biomedi-
cal engineering= Shengwu yixue gongchengxue zazhi,
29(2):206–211.
Hyv
¨
arinen, A. and Oja, E. (2000). Independent compo-
nent analysis: algorithms and applications. Neural
networks, 13(4):411–430.
Jurysta, F., Van De Borne, P., Migeotte, P.-F., Dumont,
M., Lanquart, J.-P., Degaute, J.-P., and Linkowski, P.
(2003). A study of the dynamic interactions between
sleep eeg and heart rate variability in healthy young
men. Clinical neurophysiology, 114(11):2146–2155.
Kevric, J. and Subasi, A. (2017). Comparison of signal de-
composition methods in classification of eeg signals
for motor-imagery bci system. Biomedical Signal Pro-
cessing and Control, 31:398–406.
Kim, D.-K., Lee, K.-M., Kim, J., Whang, M.-C., and Kang,
S. W. (2013). Dynamic correlations between heart and
brain rhythm during autogenic meditation. Frontiers
in human neuroscience, 7.
Klem, G. H., L
¨
uders, H. O., Jasper, H., Elger, C., et al.
(1999). The ten-twenty electrode system of the inter-
national federation. Electroencephalogr Clin Neuro-
physiol, 52(3):3–6.
An Investigation of How Wavelet Transform Can Affect the Correlation Performance of Biomedical Signals - The Correlation of EEG and
HRV Frequency Bands in the Frontal Lobe of the Brain
145

Kumari, P., Kumar, S., and Vaish, A. (2014). Feature ex-
traction using emprical mode decomposition for bio-
metric system. In Signal Propagation and Computer
Technology (ICSPCT), 2014 International Conference
on, pages 283–287. IEEE.
Kutlu, Y. and Kuntalp, D. (2012). Feature extraction for
ecg heartbeats using higher order statistics of wpd
coefficients. Computer methods and programs in
biomedicine, 105(3):257–267.
Lane, R., Reiman, E., Ahern, G., and Thayer, J. (2001).
21. activity in medial prefrontal cortex correlates with
vagal component of heart rate variability during emo-
tion. Brain and Cognition, 47(1-2):97–100.
Lewis, N. G., McGovern, J. B., Miller, J. C., Eddy,
D. R., and Forster, E. M. (1988). Eeg indices of
g-induced loss of consciousness (g-loc). Techni-
cal report, SCHOOL OF AEROSPACE MEDICINE
BROOKS AFB TX.
Lin, C.-W., Wang, J.-S., and Chung, P.-C. (2010). Min-
ing physiological conditions from heart rate variabil-
ity analysis. IEEE Computational Intelligence Maga-
zine, 5(1):50–58.
Liou, L.-M., Ruge, D., Kuo, M.-C., Tsai, J.-C., Lin, C.-
W., Wu, M.-N., Hsu, C.-Y., and Lai, C.-L. (2014).
Functional connectivity between parietal cortex and
the cardiac autonomic system in uremics. The Kaoh-
siung journal of medical sciences, 30(3):125–132.
McCraty, R., Atkinson, M., Tomasino, D., and Bradley,
R. T. (2009). The coherent heart heart-brain interac-
tions, psychophysiological coherence, and the emer-
gence of system-wide order. Integral Review: A
Transdisciplinary & Transcultural Journal for New
Thought, Research, & Praxis, 5(2).
Mirsadeghi, M., Behnam, H., Shalbaf, R., and Moghadam,
H. J. (2016). Characterizing awake and anesthetized
states using a dimensionality reduction method. Jour-
nal of Medical Systems, 40(1):1.
Miyashita, T., Ogawa, K., Itoh, H., Arai, Y., Ashidagawa,
M., Uchiyama, M., Koide, Y., Andoh, T., and Yamada,
Y. (2003). Spectral analyses of electroencephalogra-
phy and heart rate variability during sleep in normal
subjects. Autonomic Neuroscience, 103(1):114–120.
Mporas, I., Tsirka, V., Zacharaki, E. I., Koutroumani-
dis, M., Richardson, M., and Megalooikonomou, V.
(2015). Seizure detection using eeg and ecg signals
for computer-based monitoring, analysis and manage-
ment of epileptic patients. Expert Systems with Appli-
cations, 42(6):3227–3233.
Mumtaz, W., Xia, L., Yasin, M. A. M., Ali, S. S. A., and
Malik, A. S. (2017). A wavelet-based technique to
predict treatment outcome for major depressive disor-
der. PloS one, 12(2):e0171409.
Nasehi, S. and Pourghassem, H. (2011). Real-time seizure
detection based on eeg and ecg fused features using
gabor functions. In Intelligent Computation and Bio-
Medical Instrumentation (ICBMI), 2011 International
Conference on, pages 204–207. IEEE.
Prinsloo, G. E., Rauch, H. L., Karpul, D., and Derman,
W. E. (2013). The effect of a single session of short
duration heart rate variability biofeedback on eeg: a
pilot study. Applied psychophysiology and biofeed-
back, 38(1):45–56.
Sakai, S., Hori, E., Umeno, K., Kitabayashi, N., Ono,
T., and Nishijo, H. (2007). Specific acupunc-
ture sensation correlates with eegs and autonomic
changes in human subjects. Autonomic Neuroscience,
133(2):158–169.
Saper, C. B., Scammell, T. E., and Lu, J. (2005). Hypothala-
mic regulation of sleep and circadian rhythms. Nature,
437(7063):1257.
Stoica, P. and Moses, R. L. (1997). Introduction to spectral
analysis, volume 1. Prentice hall Upper Saddle River,
NJ.
Stuss, D. T. and Benson, D. F. (1986). The frontal lobes.
Raven Pr.
Sudarshan, V. K., Acharya, U. R., Oh, S. L., Adam, M.,
Tan, J. H., Chua, C. K., Chua, K. P., and San Tan,
R. (2017). Automated diagnosis of congestive heart
failure using dual tree complex wavelet transform and
statistical features extracted from 2s of ecg signals.
Computers in Biology and Medicine, 83:48–58.
Takahashi, T., Murata, T., Hamada, T., Omori, M., Kosaka,
H., Kikuchi, M., Yoshida, H., and Wada, Y. (2005).
Changes in eeg and autonomic nervous activity dur-
ing meditation and their association with personal-
ity traits. International Journal of Psychophysiology,
55(2):199–207.
Thomas, M., Das, M. K., and Ari, S. (2015). Automatic
ecg arrhythmia classification using dual tree complex
wavelet based features. AEU-International Journal of
Electronics and Communications, 69(4):715–721.
Thomas, P. and Moni, R. (2016). Methods for improving the
classification accuracy of biomedical signals based on
spectral features. Technology, 7(1):105–116.
Triggiani, A. I., Valenzano, A., Del Percio, C., Marzano,
N., Soricelli, A., Petito, A., Bellomo, A., Bas¸ar, E.,
Mundi, C., Cibelli, G., et al. (2016). Resting state
rolandic mu rhythms are related to activity of sym-
pathetic component of autonomic nervous system in
healthy humans. International Journal of Psychophys-
iology, 103:79–87.
Unser, M. and Aldroubi, A. (1996). A review of wavelets
in biomedical applications. Proceedings of the IEEE,
84(4):626–638.
Valderrama, M., Alvarado, C., Nikolopoulos, S., Mar-
tinerie, J., Adam, C., Navarro, V., and Le Van Quyen,
M. (2012). Identifying an increased risk of epileptic
seizures using a multi-feature eeg–ecg classification.
Biomedical Signal Processing and Control, 7(3):237–
244.
Yang, C. C., Lai, C.-W., Lai, H. Y., and Kuo, T. B.
(2002). Relationship between electroencephalogram
slow-wave magnitude and heart rate variability during
sleep in humans. Neuroscience letters, 329(2):213–
216.
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
146
