
Identifying Isolated Glioblastoma Tissues in Human Patients through
Their Optical and Spectral Properties
Hussein Mehidine
1,5
, Fanny Poulon
1
, Ali Ibrahim
1
, Marjorie Juchaux
1
, Pascale Varlet
2,3
,
Bertrand Devaux
4
, Johan Pallud
3,4
and Darine Abi Haidar
1,5
1
Laboratory, UMR 8165-CNRS/IN2P3, Paris-Saclay University, 91405 Orsay, France
2
Neuropathology Department, Sainte-Anne Hospital, 75014 Paris, France
3
IMA BRAIN, INSERMU894, Centre de Psychiatrie et de Neurosciences, Paris, France
4
Neurosurgery Department, Sainte-Anne Hospital, 75014 Paris, France
5
Université Paris Diderot, Sorbonne Paris Cité, F-75013 Paris, France
Keywords: Glioblastoma, Scattering Coefficient, Absorption Coefficient, Endogenous Fluorescence.
Abstract: Survival rates and health-related quality of life of adult patients suffering from glioblastoma depend
significantly on the extent (no residual tumor tissue) and precision (no collateral damage) of the surgical
resection. Assistance in defining the borders of the infiltrating component of the glioblastoma would be
valuable to improve outcomes. A tissue can be defined by its optical properties : absorption, scattering,
intensity of fluorescence, that will give a unique signature. In this work we look at the absorption and
scattering coefficients of glioblastoma and control tissues from adult patients using an integrating sphere,
spectral measurements were also took on the samples using a fiber endoscope. The preliminary results show
the potential of using endogenous fluorescence for intraoperative identification of residual glioblastoma
tissue in the wall of the surgical cavity of resection.
1 INTRODUCTION
Glioblastoma (GBM) is the most common and most
aggressive malignant primary brain tumor in adults.
Following a magnetic resonance imaging (MRI)
analysis, its oncological treatment comprises 1) a
maximal safe resection encompassing the contrast-
enhanced tumor tissue, whenever feasible, 2) an
adjuvant treatment with combined radiotherapy and
concomittant chemotherapy followed by adjuvant
chemotherapy. The surgical resection technique is
limited by the difficulty to discriminate
intraoperatively between healthy tissue and tissue
infiltrated by isolated glioblastoma cells, mainly in
the wall of the surgical resection cavity, which
contain the infiltrated boundaries of the tumor. The
clinical concern is that, because glioblastoma
infiltration is not completly resected, the tumor
recure systematically and the patient will have to
return for a new operation which could lead to more
constraining side effects and reduce chances of
survival. The median overall survival is less than 18
months.
Indeed, glioblastoma is one of the most
infiltrating tumor, even if the solid tumor area is
easily detectable on MRI as a contrast-enhanced
tumor tissue, the infiltrated regions, which contains
active and isolated glioblastoma cells, are not
contrasted from healthy regions. These infiltrated
area have the same visual appearance as the healthy
ones, which makes them difficult to delineate.
Nowadays, the only technique that gives accurate
information on infiltrated area is the
histolopathogical analysis of a biopsy sample, which
is done ex-vivo and takes several days.
Recently, several intraoperative techniques have
been proposed to solve this problem, such as the
linear endomicroscope commercialized by Mauna
Kea technologies. This tool presents the advantages
of in-vivo and real time imaging. However, this
technique still not able to provide a multimodality of
measurements, which seems necessary to
discriminate healthy from tumoral tissues with a
strong relialability.
Our team develops a non-limear optical
endomicroscope, which is a new tool designed to the
surgical room. This intraoperative system will allow
136
Mehidine, H., Poulon, F., Ibrahim, A., Juchaux, M., Varlet, P., Devaux, B., Pallud, J. and Haidar, D.
Identifying Isolated Glioblastoma Tissues in Human Patients through Their Optical and Spectral Properties.
DOI: 10.5220/0006531501360140
In Proceedings of the 6th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2018), pages 136-140
ISBN: 978-989-758-286-8
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
the neurosurgeon to have a fast, reliable and
reproducible response on the nature of surrounding
tumor tissues, using several endogenous contrasts.
The multimodaly, regrouping Two Photons
Excitation (TPEF), Second Harmonic Generation
(SHG), spectral and fluorescence lifetime
measurements, was already proved as essential in
glioma tissue detection (Zanello et al., 2017).
In this context, it is necessary to identify all optical
properties of glioblastoma tumor, and compare them
with those of healthy tissues. In this work, many
optical parameters of frozen samples were found,
absorption and scattering coefficients using the
integrated sphere technique under 430 nm. The
spectral signature of endogenous fluorophores, using
linear excitation at 375 nm and 405 nm excitation
wavelength, were recorded.
2 MATERIALS AND METHODS
2.1 Samples
Samples were carried from the neurosurgery
department to the neuropathology laboratory at
Sainte-Anne hospital, Paris. The delay between the
end of the resection operation and the reception of
tissue was about fifteen minutes. After that, the
samples were stored at -80°C for few days. Then
they were cut at -18°C into 600 µm thickness
sections using a cryostat (Leica Microsystems) and
fixed with a 100% alcohol solution. Six
glioblastomas and seven healthy cortex samples
were analysed in this preliminary study.
2.2 Spectral, Transmittance and
Reflectance Study
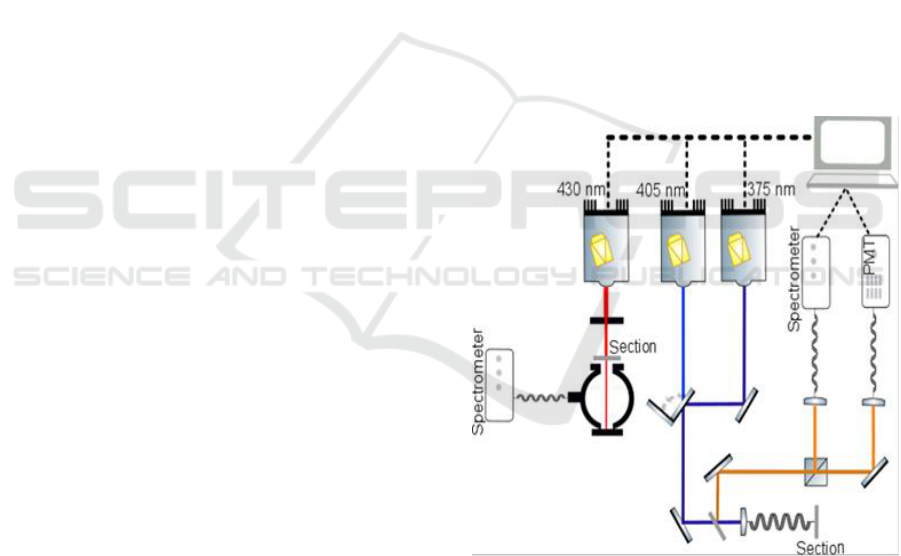
The set-up shown in figure 1, is placed in the
neuropathology department at Sainte-Anne Hospital,
Paris-France. The setup is based on a linear
excitation fiber endoscope with three pulsed diode
lasers.
For integrating sphere measurements, the pulsed
diode laser emitting at 430 nm (Picoquant Germany,
LDH-P-C-430B, FWHM 70 ps) excitation
wavelength was used. Transmittance and reflectance
measurements of each sample were achieved using
an integrating sphere (Thorlabs, IS200-4). Its inner
surface is covered by a 99% reflective teflon, it have
four ports with a 12.7 mm diameter, and a fifth port
with a 3mm diameter to transfer the signal from the
integrating sphere to the spectrometer (Ocean optics,
HR2000) using an optical fiber (Ocean optics).
The average laser power used for this study was
around 5 mW. A diaphragm was placed after the
laser source to reduce the beam diameter to 1 mm
and the laser beam was pointed to five differents
Region Of Interest (ROI) in each specimen.
Transmittance and reflectance were measured for
each ROI, and finally the average of these five
values was calculated.
For spectral measurements, the excitation is
performed with the two others pulsed diode lasers
emitting at 405 nm (Picoquant-Germany, LDH-P-C-
405B, FWHM 60 ps,) and 375 nm (PicoQuant-
Germany, LDH-P-C-375B, FWHM 45ps) with a
maximal power of 1.1 mW. These diodes were
controlled with a driver (PicoQuant-Germany, PDL-
808 “Sepia”).
A specific Photonic Crystal Double-Clad Fiber
(PC-DCF) was used to excite and collect the
fluorescence signal (Ibrahim et al., 2016a, 2016b).
Collected signal is lead to the spectrometer (Ocean
Optics, QEPro 6500) through a long pass filter
(Semrock, SR420) to eliminate laser reflection.
Figure 1: Schematic representing the implemented setup,
including the integrating sphere and the spectral analysis.
3 DATA ANALYSIS
3.1 Optical Coefficients
The Inverse Adding Doubling (IAD) algorithm (S.
A. Prahl et al., 1993) was used to find the absorption
coefficient (µ
a
) and the reduced scattering
Identifying Isolated Glioblastoma Tissues in Human Patients through Their Optical and Spectral Properties
137
coefficient (µ’
s
) by refearing to the measured values
of the transmittance and the reflectance.
The scattering coefficient µ
s
is deduced using the
equation (1)
µ
s
= µ’
s
/(1-g)
(1)
Where g is the anisotropy factor of the sample,
so we consider that g=0.89 for glioblastoma samples
and g=0.86 for cortex samples (Poulon et al., 2017).
This algorithm solves iteratively the radiative
transport equation until the numerical adjustment
and the experimental values of reflectance and
transmittance matches (Prahl et al., 1993). The
refractive index of the samples was not measured:
we consider that the refractive index n = 1.44 is the
same for all samples examinated by refering to the
literature (Bevilacqua et al., 1999).
3.2 Spectral Analysis
A Matlab code developed by our team and already
used in previous publications (Haidar et al., 2015)
(Zanello et al., 2017) was used to process spectral
data.
During spectral measurements, we used two
excitation wavelengths: (i) 405 nm to excite
efficiently five different endogenous fluorophores:
Nicotinamide Adenine Dinucleotide (NADH),
Flavins (FAD), lipopigments, porphyrins and
chlorines, and (ii) 375 nm to excite effectively the
NADH and FAD.
4 RESULTS
4.1 Optical Coefficients
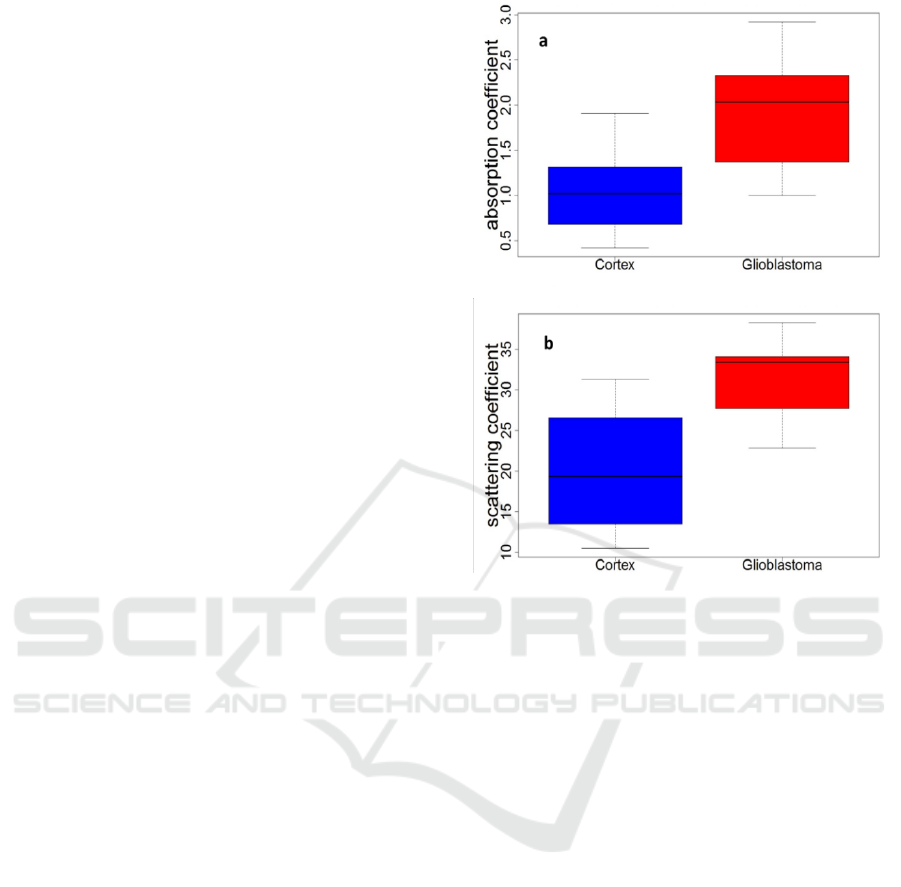
The distribution of all scattering coefficient µ
s
values obtained in our measurements are shown in
Figure 2. The glioblastoma samples presented a
higher scattering coefficient than the cortex samples
and the glioblastoma samples had a higher
absorption coefficient than the cortex samples.
Figure 2: Distribution of the scattering coefficient values
(mm
-1
)(a) and the absorption coefficient values (mm
-1
) (b)
for glioblastoma and cortex tissues excited with 430 nm.
4.2 Spectral Measurements
In Figure 3, we compared the total fluorescence
intensity of glioblastoma and cortex samples, excited
with 405 nm (a) and 375 nm (b).
The difference in the spectral intensity was well
underlined between glioblastoma and cortex
samples. The emitted fluorescence from cortex
samples was 2.5 times higher than for glioblastoma
samples using a 405 nm excitation wavelength, and
2 times higher using 375 nm. We observed that
using 375 nm excitation wavelength we optimized
the NADH excitation and so the emitted
fluorescence signal was higher at this wavelength.
Using 405 excitation molecule we excited five
endogenous molecules (Zanello et al., 2016) as
shown in the spectral shape of figure 3.a.
PHOTOPTICS 2018 - 6th International Conference on Photonics, Optics and Laser Technology
138
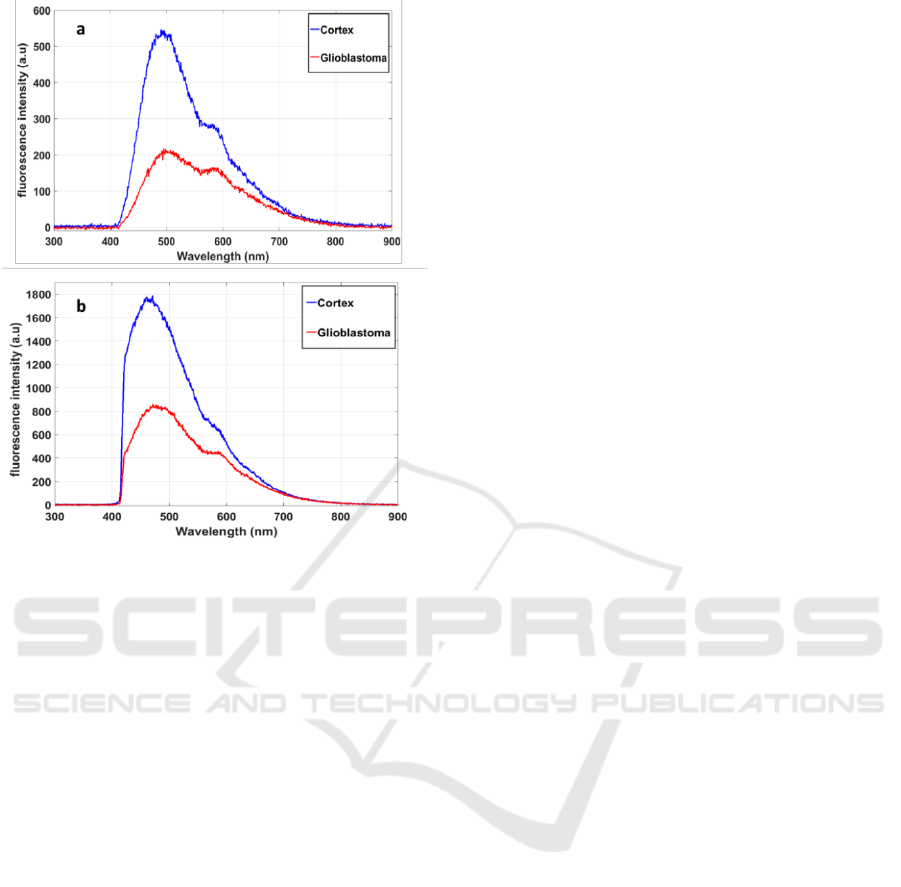
Figure 3: Fluorescence spectra of glioblastoma and cortex
tissues excited with 405 nm (a) and 375nm (b)
wavelength.
5 DISCUSSION
In this preliminary study, we compared fixed cortex
and glioblastoma samples from adult patients using
two types of measurements. First, we observed that
the scattering and absorption coefficient are higher
in glioblastoma samples than in cortex samples. This
could be related to the fact that glioblastoma tissues
have denser vascularization and more collagen fibres
than cortex. This vascularization could be the source
of light scattering in such tissues. Added to the fact
that glioblastoma tissues contain neovascularization
which could affect the absorption coefficient.
By referring to our previous spectral studies on
rats brain tumors and healthy tissues (Haidar et al.,
2015) and on human brain tissues (Zanello et al.,
2017) using 405 nm excitation wavelength, the five
excited fluorophores are more concentrated in the
healthy tissues than in the glioblastoma tissues. For
that, the total fluorescence signal in cortex tissue is
higher than in glioblastoma. The same trend was
shown using 375 nm excitation. At this excitation
wavelength the absorption efficient section of
NADH and the FAD is higher than at 405nm
excitation wavelength. So, we optimise the
efficiency of excitation of this two endogenous
molecules and specially the excitation of NADH.
We can show also that endogenous fluorescence
from healthy tissues is higher than glioblastoma
tissues. This observation is of paramount important
as spectral response can discriminate healthy from
glioblastoma tissues. This is in accordance with the
literature of spectral analysis on freshly resected
tissues (Zanello et al., 2017).
Here, we show bimodal quantitative
measurements on the same tissues. These
measurements prove the power of optical detection,
based on endogenous fluorescence of frozen tissues
to discriminate healthy from tumoral tissues.
This opens a promising door in the detection of
tumors margins. It proved also how important is the
multimodality to detect with reliability the nature
tissues area, highlighting the importance of our
optical endomicroscope.
In the future studies, we will extend our cohort
by examining more types of human brain tumors and
also extend our study to freshly extracted samples.
ACKNOWLEDGEMENTS
This work is supported by a Plan Cancer with
Physicancer program grant “IMOP,” a “Défi
instrumental” program grant from CNRS, the Institut
National de Physique Nucléaire et de Physique des
Particules (IN2P3), and the “Ligue contre le cancer”.
REFERENCES
Bevilacqua, F., Piguet, D., Marquet, P., Gross, J.D.,
Tromberg, B.J., Depeursinge, C., 1999. In vivo local
determination of tissue optical properties:
applications to human brain. Appl. Opt. 38, 4939–
4950.
Haidar, D.A., Leh, B., Zanello, M., Siebert, R., 2015.
Spectral and lifetime domain measurements of rat
brain tumors. Biomed. Opt. Express 6, 1219–1233.
Ibrahim, A., Poulon, F., Habert, R., Lefort, C., Kudlinski,
A., Haidar, D.A., 2016a. Characterization of fiber
ultrashort pulse delivery for nonlinear
endomicroscopy. Opt. Express 24, 12515–12523.
Ibrahim, A., Poulon, F., Melouki, F., Zanello, M., Varlet,
P., Habert, R., Devaux, B., Kudlinski, A., Haidar,
D.A., 2016b. Spectral and fluorescence lifetime
endoscopic system using a double-clad photonic
crystal fiber. Opt. Lett. 41, 5214–5217.
Poulon, F., Mehidine, H., Juchaux, M., Varlet, P., Devaux,
B., Pallud, J., Abi Haidar, D., 2017. Optical
properties, spectral, and lifetime measurements of
Identifying Isolated Glioblastoma Tissues in Human Patients through Their Optical and Spectral Properties
139

central nervous system tumors in humans. Sci. Rep. 7.
doi:10.1038/s41598-017-14381-1.
Prahl, S.A., van Gemert, M.J., Welch, A.J., 1993.
Determining the optical properties of turbid media by
using the adding–doubling method. Appl. Opt. 32,
559–568.
Zanello, M., Poulon, F., Pallud, J., Varlet, P., Hamzeh, H.,
Lahoud, G.A., Andreiuolo, F., Ibrahim, A., Pages, M.,
Chretien, F., 2017. Multimodal optical analysis
discriminates freshly extracted human sample of
gliomas, metastases and meningiomas from their
appropriate controls. Sci. Rep. 7, 41724.
Zanello, M., Poulon, F., Varlet, P., Chretien, F.,
Andreiuolo, F., Ibrahim, A., Pallud, J., Dezamis, E.,
Abi‐Lahoud, G., Nataf, F., 2016. Multimodal optical
analysis of meningioma and comparison with
histopathology. J. Biophotonics.
PHOTOPTICS 2018 - 6th International Conference on Photonics, Optics and Laser Technology
140
