
An Adaptive Scrum Model for Developing Disease Registries
Hatem Bellaaj
1,3
, Afef Mdhaffar
2,3
, Mohamed Jmaiel
3
and Bernd Freisleben
4
1
Department of Mathematics and Computer Science, IPEIS, University of Sfax, Sfax, Tunisia
2
Department of Mathematics and Computer Science, ISSAT, University of Sousse, Sousse, Tunisia
3
Digital Research Center of Sfax, Sfax, Tunisia
4
Department of Mathematics and Computer Science, University of Marburg, Marburg, Germany
Keywords:
Disease Registry, Agile, Scrum, Sprint, Transparency, Inspection, Adaptation.
Abstract:
This paper presents an adaptive model for developing disease registries. The proposed model is based on the
Scrum methodology. It can be used to draw a road map to identify priorities, inputs, outputs, team members
and exchanges for all tasks required to develop disease registries. Our model has been applied to real cases
from several Tunisian hospitals where it has improved the efficiency of the team members. The developed
disease registries are currently used in Tunisia. They allow medical researchers to identify origins of diseases,
establish new protocols, perform surveys and compute morbidity.
1 INTRODUCTION
Disease registries are used to collect and analyze
epidemiological information related to the frequency
and distribution (i.e., incidence and prevalence) of
a specific disease. In addition to the detection of
known symptoms and diagnosis parameters of dis-
eases, statistics obtained from disease registries can
help doctors to discover new risk factors. These are
important to assess the medical situation and to pro-
vide framework conditions, preventive measures and
management plans. A clinical information system
refers to the organization of clinical data from med-
ical records of patients to coordinate the delivery of
interventions and self-management support activities
for the entire population. Building a disease registry
is crucial for managing a population of patients suffer-
ing from chronic diseases (McCulloch et al., 1998).
A disease registry can be considered as a medi-
cal information system (MIS) and as an instance of a
general information system (IS). The development of
this kind of software system is a resource-intensive
process that involves different groups of people in
an organization. Several software development mod-
els have emerged. Among them are: the systems-
development life cycle (SDLC), rapid application de-
velopment (RAD), and agile methodologies. Agile
methodoloies are widely used for software project
management, and Scrum is the most common agile
method (Schwaber and Sutherland, 2001).
In this paper, we present a new model for devel-
oping disease registries based on Scrum. The model
is adaptive in the sense that it provides support for
incorporating recommendations that satisfy specific
requirements of this domain. The proposed model
has been applied to real cases from several hospi-
tals in Tunisia, such as the Tunisian Fanconi Anemia
Registry, the Tunisian Gaucher Disease Registry and
the Tunisian Non-Hodgkin’s Lymphoma Registry. In
these projects, the use of our model has (a) acceler-
ated the establishment of paper-based disease forms
as functional specifications, (b) established a strong
relation with the doctors, (c) minimized losses due to
changes in fields’ data types by fixing the sprint dura-
tion between 7 to 15 days, (d) found the right balance
between documentation and discussion, (e) increased
chances that the final product is as originally speci-
fied.
The paper is organized as follows. Section 2 intro-
duces disease registries. Section 3 discusses related
work. In Section 4, we summarize Scrum. Section 5
presents the proposed methodology. Applications are
described in Section 6. Section 7 concludes the paper.
2 DISEASE REGISTRIES
A disease registry stores medical information related
to the same disease. This allows medical researchers
484
Bellaaj H., Mdhaffar A., Jmaiel M. and Freisleben B.
An Adaptive Scrum Model for Developing Disease Registries.
DOI: 10.5220/0006297804840491
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 484-491
ISBN: 978-989-758-213-4
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

to (1) review and study health records of many in-
dividuals, (2) answer questions about diagnostic dis-
ease parameters and (3) build a knowledge database
for each disease.
A disease registry is a tool for tracking the clin-
ical care and outcomes of a defined patient popula-
tion. Most disease registries are used to support care
management for groups of patients suffering from one
or many chronic diseases, such as diabetes, coronary
artery disease and asthma. In contrast to paper-based
registries that have been used to track patients suffer-
ing from chronic diseases, digital registries provide
users with an automated way to (1) store data, (2) cre-
ate, sort, and display lists of patients and (3) extract
data used for planning, quality improvement, report-
ing, and direct care delivery (Hummel, 2000).
Digital disease registry functionality is included
(i.e., available as an add-on) in electronic health
record (EHR) products. In addition, stand-alone op-
tions can be implemented and are usually simpler to
set up than EHRs. Based on their priorities, some
organizations may choose to implement digital dis-
ease registries as an interim step prior to implement-
ing a more comprehensive EHR system. Disease reg-
istries can also provide more flexibility for reporting
and aggregating data from multiple data sources. To
implement a digital disease registry, an organization
needs to analyze and adjust practice workflows to sup-
port new data collection requirements and to integrate
the new information from their registry into decision
making and planning (Hummel, 2000).
3 RELATED WORK
3.1 The Process
A software development methodology is used to
structure, plan, and control the process of develop-
ing an information system. Several methodologies to
develop software have been proposed, such as Agile
Software Development, Crystal Methods, Dynamic
Systems Development Model (DSDM), Extreme Pro-
gramming (XP), Feature Driven Development (FDD),
Joint Application Development (JAD), Lean Develop-
ment (LD), Rapid Application Development (RAD),
Rational Unified Process (RUP), Scrum, Spiral, and
Systems Development Life Cycle (SDLC). Three of-
ten used methods are discussed below.
3.1.1 Systems Development Life Cycle (SDLC)
SDLC is considered to be the oldest project execu-
tion framework. It can be used to manage large soft-
ware projects, associated with corporate systems run-
ning on mainframes. It is a structured methodology,
designed to manage complex projects that implicate
many programmers and systems, having a high im-
pact on the organization (Bourgeois, 2016).
3.1.2 Rapid Application Development (RAD)
RAD is a software development methodology that
favors rapid prototyping on complex planning. In
RAD, modules are developed in parallel as proto-
types. Later, they are merged to the final product
for faster delivery (Vickoff, 2000). The RAD method
presents a secured and short development cycle fol-
lowing different phases: framing, design, building
with fixed duration. Each phase takes about 90 to 120
days. RAD includes methods, techniques and tools
to achieve four potentially contradictory objectives:
budget, deadlines, technical quality, functional qual-
ity and visibility (Vickoff, 2000). The most important
aspect for this model to be successful is to make sure
that developed prototypes are reusable.
3.1.3 Agile Methods
Agile methods aim to reduce the life cycle of soft-
ware development by creating a minimal version and
then integrating functionality by an iterative process
based on a customer listening and tests throughout
the development cycle. The origin of agile methods is
linked to the instability of the technological environ-
ment and the fact that the client is often unable to de-
fine his or her needs exhaustively from the beginning
of the project. The term ”agile” thus refers to the abil-
ity to adapt to changes in the context and changes in
specifications occurring during the development pro-
cess. It was first coined in 2001 in the Manifesto
for Agile Software Development (Agile Manifesto)
(Beck et al., 2001). Now, agile refers to any process
that follows concepts of the Agile Manifesto. There
are four main points in the Agile Manifesto:
1. Individuals and interactions over processes and
tools
2. Working software over comprehensive documen-
tation
3. Customer collaboration over contract negotiation
4. Responding to change over following a plan
The Agile Manifesto lists 12 principles to guide
teams on how to execute with agility. The principles
are described below.
1. Our highest priority is to satisfy the customer
through early and continuous delivery of valuable
software.
An Adaptive Scrum Model for Developing Disease Registries
485

2. Welcome changing requirements, even late in de-
velopment. Agile processes harness change for
the customers competitive advantage.
3. Deliver working software frequently, from a cou-
ple of weeks to a couple of months, with prefer-
ence to the shorter timescale.
4. Business people and developers must work to-
gether daily throughout the project.
5. Build projects around motivated individuals. Give
them the environment and support their need, and
trust them to get the job done.
6. The most efficient and effective method of convey-
ing information to and within a development team
is face-to-face conversation.
7. Working software is the primary measure of
progress.
8. Agile processes promote sustainable develop-
ment. The sponsors, developers, and users should
be able to maintain a constant pace indefinitely.
9. Continuous attention to technical excellence and
good design enhances agility.
10. Simplicity: the art of maximizing the amount of
work not done – is essential.
11. The best architectures, requirements, and designs
emerge from self-organizing teams.
12. At regular intervals, the team reflects on how to
become more effective, then tunes and adjusts its
behavior accordingly (Beck et al., 2001).
3.2 The Product
The basic architecture of a digital disease registry
consists of four layers. They are described below.
3.2.1 Data Layer
Data is stored in a specific electronic health record
(EHR), with data corresponding to parameters related
to the diagnosis of the disease, such as responses to a
treatment. The included data respects specific proper-
ties, such as persistence, accuracy (Staroselsky et al.,
2008) and validity (Carroll et al., 2007). There are
three main ways to populate the registry with data:
1. directly through the fields in the disease form.
2. importing data using standard interoperability
mechanisms, such as HL7 or CCD. Data is au-
tomatically inserted into the database.
3. combining these two approaches.
The data layer must respect the international stan-
dards. Among them are:
• ISO 13119:2012 describes metadata that re-
lates to resources including medical knowledge.
This standard applies mainly to digital docu-
ments, such as WWW resources, accessible from
databases or file transfers. It can also be applied
to paper documents, such as articles in the medi-
cal literature (ISO 13119, 2012).
• ISO 13606-1:2008 describes the communication
of some or all of the electronic health record
(EHR). The record of a patient is identified be-
tween the DSEs, or between this latest and a cen-
tralized repository. It can be used to communi-
cate an EHR system or repository with clinical ap-
plications or middleware components that need to
access or provide EHR data (ISO 13606-1, 2008).
3.2.2 Security Layer
We distinguish between different kinds of users: sys-
tem administrator, group administrator (who estab-
lishes the disease registry form, statistics parameters,
therapy protocol, etc.), reference doctors, participant
doctors, analysts, developers, patients, simple users,
etc. A set of rules that defines the behavior of each
kind of users must be specified at the start of the
project. Different security norms are defined for such
systems. Two of them are described below:
• ISO / TR 11633-1, 2009 concerns remote main-
tenance services (RMS) for information systems
in healthcare facilities. It presents an example
of a risk analysis that protects the information in
the medical information system (ISO/TR 11633-
1, 2009).
• ISO / TS 14441, 2013 examines the electronic pa-
tient records systems at the point of clinical care
that are also interoperable with EHRs. It treats
their protections in terms of security and privacy
through a set of security and privacy rules. It in-
cludes guidelines and practices for conformity as-
sessment (ISO/TS 14441, 2013).
3.2.3 Dashboard Layer
The dashboard layer is the main goal of disease reg-
istries. It shows morbidity, mortality, survey curves,
treatment responses and illness origins. The plurality
of dashboard components is associated with a plural-
ity of types of health-care content and are based on
parameters received from a user.
3.2.4 Interoperability Layer
Interoperability consists of communication between
healthcare organizations. The medical information
HEALTHINF 2017 - 10th International Conference on Health Informatics
486

system is specific for each organization. Building a
global medical information system for many organi-
sations is a complex task. Indeed, there is an array of
healthcare-related applications that supports multiple
needs but remains isolated or incompatible. Thus, in-
teroperability is a challenge (Hammami et al., 2014).
Different interoperability norms are defined for such
systems. Some of them are presented below.
• ISO / TR 16056-1, 2004 introduces the interoper-
ability of telehealth systems and networks. It also
defines telehealth and related terms.
• ISO EN 13606: Medical record communication is
based on a two-model approach using paradigms.
This ensures that the data collected from heteroge-
neous systems are correctly interpreted. The dual
model provides the basis for a stable architecture.
It separates information from knowledge.
4 SCRUM
Scrum originates from the sporting term rugby mean-
ing: melee. Like this technical aspect of the game, the
methodology asks its actors to be united in the accom-
plishment of a project, in achieving a goal. A melee
is not a unique process. This is a part of the game
that is often found to move the team forward. In the
same concept Scrum uses a procedure that we name
sprint. Each iteration or sprint provides a functional
part of product. Three pillars uphold every implemen-
tation of empirical process control: transparency, in-
spection, and adaptation (Schwaber and Sutherland,
2001). Scrum includes definitions, descriptions, con-
cepts and methods for better running projects. It de-
fines details for: the Scrum team, the product owner
(PO), the development team, the Scrum master, the
Scrum events, the sprint, the daily Scrum, the sprint
review, the sprint retrospective, the artifacts, the prod-
uct backlog, the sprint backlog and the artifact trans-
parency.
5 AN ADAPTIVE SCRUM MODEL
FOR DISEASE REGISTRIES
This section presents all adapted pillars, mechanisms,
and concepts for developing disease registries using
the Scrum model.
5.1 Three Pillars
5.1.1 Transparency
Transparency insurance is difficult between people
who do not share the same discourse. Thus, we pro-
pose to build:
• a medical dictionary, including term definitions
comprehensible by all players
• a crossing table between the variables, specifying:
mathematical equations, if they exist and logical
relations between values, e.g., it is not logical to
find the symptom S1 true while the variable V1 is
normal
• an effective way to present a variable: selection
between alphanumeric value, checkbox, text field
to be specified
5.1.2 Inspection
There are three levels of inspections that can be done
sequentially and at the same time according to the it-
eration in question:
• functional validation by computer scientists
• medical validation of the distribution of the fields
to be entered, the logical and mathematical links
of the different variables
• validation of the possibility of statistical interpre-
tation of the different variables
The second type of validation often leads to the
addition, deletion or modification of the type of pre-
sentation of some fields. Development constraints
cause developers to modify a type of representation
or an operating logic that can essentially lead to poor
statistical quality. Inspection becomes foolish and can
greatly slow down the development process.
5.1.3 Adaptation
Scrum prescribes four formal events for inspection
and adaptation: sprint planning, daily scrum, sprint
review and sprint retrospective. The fact that the
project is carried out by people of different special-
ties, a misunderstanding of need can delay and burden
the concept of adaptation. Reports of daily meetings
at a frequency of maximally three days can be sent to
the head of the medical study group.
5.2 The Scrum Team
The Scrum team consists of a product owner (PO),
the development team, and a Scrum master. Scrum
An Adaptive Scrum Model for Developing Disease Registries
487

teams are self-organizing and cross-functional. In the
following, the needed skills, the specific mission, re-
lations and input / output of each of them are detailed.
For a disease registry, three teams should be estab-
lished, which will work together and simultaneously:
the doctors who are members of the study group,
statisticians and developers. The PO leads these three
teams and takes decisions after meetings including all
the teams or representative members of each of them.
Therefore, (s)he is the first person responsible for the
product backlog.
The team model in Scrum is designed to optimize
flexibility, creativity, and productivity. For disease
registries, flexibility is guaranteed by trust, designing,
and good communication. Trust means the capability
of achieving a good job, possibly by young employ-
ees. Designing consists of giving priority to dispatch-
ing the members of the team and not the tasks. Pro-
ductivity is increased by communication (e.g., regular
meetings), tools (e.g., management version applica-
tions) and documentations.
Scrum teams deliver products iteratively and in-
crementally, maximizing opportunities for feedback.
An average incremental delivery period can be de-
fined as about 10% of the number of fields for form
and dashboard pages. For the user management mod-
ule, 25% of the total number of user groups is appreci-
ated. For other modules, these values must be defined
at the start of the project but cannot exceed two weeks
as a period to increase profitability.
5.2.1 The Product Owner
The product owner is in charge of maximizing the
value of the product and the work of the development
team. The PO is the sole person responsible for man-
aging the product backlog (Sverrisdottir et al., 2014).
For disease registries, it is recommended that the
product owner communicates periodically the up-
dated backlog to the leader of the disease study group,
discusses and may change some priorities.
The PO insures that the product backlog is vis-
ible, transparent, and clear to all, and shows what
the Scrum team will work on in the next step. The
draft version of backlog should be validated at the
last meeting with doctors before the kick-off of the
project.
5.2.2 The Development Team
The development team consists of professionals who
are in charge of delivering a feasible increment of the
done product at the end of each sprint. Only members
of the development team create the increment.
In Scrum, it is recommended that the development
team includes 3 to 9 members to insure efficiency
and global efficacy. For a disease registry, we have
adopted this structure: 1 designer, 1 tester, 1 archi-
tect, 1 to 2 developers for security management and 3
to 9 Java developers.
5.2.3 The Scrum Master
The Scrum master is responsible for ensuring that
Scrum is understood and implemented. It will play
exactly the same roles as defined in the Scrum guide.
Thus, it will ensure the availability of tools necessary
for the good understanding and adherence to Scrum
for the product owner and the development team.
Ideally, the Scrum master is a member of the de-
velopment team. He or she is supposed to master no-
tions of statistics. In his or her profile, conducting
research in medical informatics or participating in a
similar project is appreciated.
5.3 The Scrum Events
In Scrum, we attempt to fix regular events and to min-
imize the need for unplanned meetings. All events
are timed, so that each event has a maximum dura-
tion. Once a sprint starts, it can not be shortened or
lengthened. Events end each time the associated goal
is reached. Appropriate time must be allocated for
each sprint to avoid having waste in the process.
5.3.1 The Sprint
The heart of Scrum is a sprint. It is a time block re-
sulting in an increment of the potentially deliverable
product. It lasts for one month or less, and a deliv-
erable product increment is created. A new sprint
starts immediately after the conclusion of the previ-
ous sprint (Schwaber and Sutherland, 2001).
Sprints contain and consist of the sprint plan-
ning, daily Scrums, the development work, the sprint
review, and the sprint retrospective (Schwaber and
Sutherland, 2001). For a medical disease registry, the
average duration of a sprint is ideally between 7 and
15 working days.
5.3.2 Sprint Planning
One of the first methods spread around the world is
the V-Cycle method. It is a project organization logic
that limits the problem of reactivity in the event of an
anomaly, limiting the return to the preceding steps. It
is represented by a V whose descending branch con-
tains all the phases of the design of the project, and
the rising branch all the stages of tests of the project.
HEALTHINF 2017 - 10th International Conference on Health Informatics
488
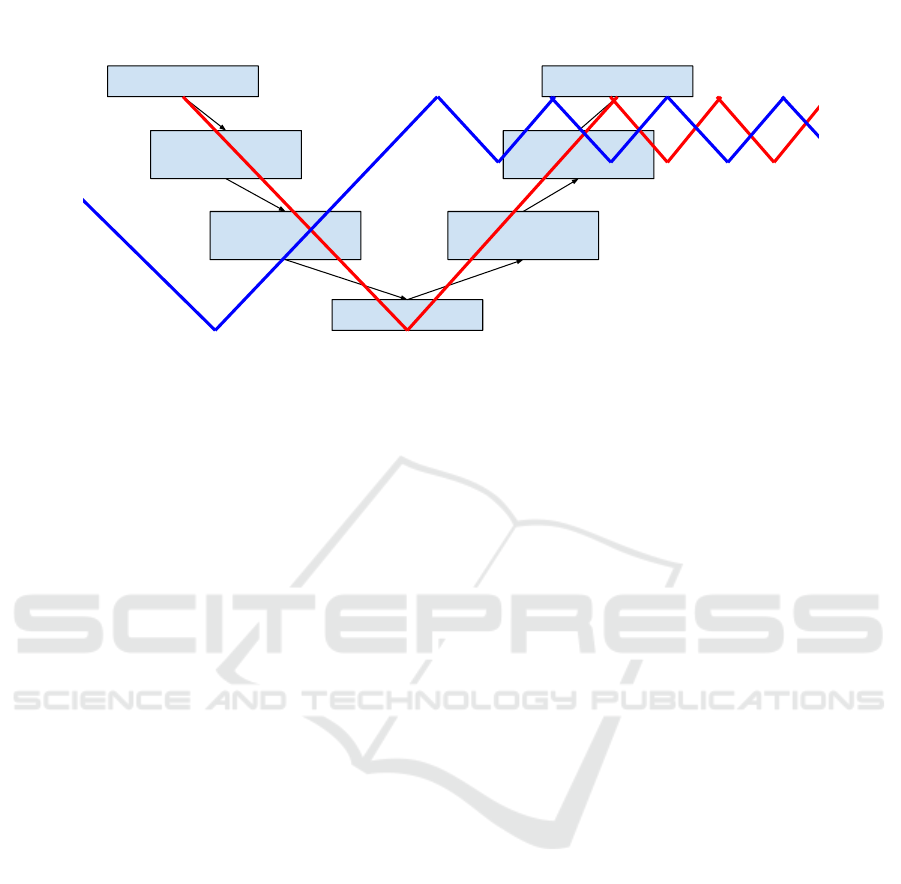
Requirements
General design
specification
Detailed design
specification
Source code
Unit testing
Component testing
Acceptance testing
Medical Disease form development
Electronic Disease form development
Figure 1: The W Model for disease registry development.
The tip of the V represents the stage of realization of
the project. Each phase of the descending branch is
related to a phase of the rising branch.
In disease registry development, we have adopted,
for the first time, this kind of project organization
method. The same thing is done with the medical
team to establish the disease form and the list of in-
cluded elements in a dashboard (statistics). For this
purpose, two V cycles (i.e., a W cycle) are estab-
lished with intersection points (see Figure 1). These
points represent meetings between members of medi-
cal and development staff. In addition to the compli-
cated problems of return in the cycle for both teams,
the meeting management presented by the intersec-
tion points in Figure 1 must be handled.
In disease registry development, we recommend
that a sprint duration is between 1 and 2 weeks. The
reduction of this value increases the profitability of
the team, but may make the management of the dif-
ferent tasks complicated. In this stage, it is important
to take into account the source version management
process. Two methods can be used: distribution ac-
cording to functionalities or according to teams. The
first is strongly recommended for disease registries.
5.3.3 Daily Scrum
The daily scrum is a 15-minute time-boxed event for
the development team to synchronize activities and
create a plan for the next 24 hours (Schwaber and
Sutherland, 2001). It is recommended that a mem-
ber of the study group participates in the daily scrum.
Since doctors are usually solicited by their patients,
it is recommended to establish these meetings at the
end of the day. Thus, a meeting should answer the
following questions:
• What did I do today that helped the development
team meet the sprint goal?
• What will I do tomorrow to help the development
team meet the sprint goal?
• Do I see any impediment that prevents me or the
development team from meeting the sprint goal?
5.3.4 Sprint Review
A sprint review is held at the end of the sprint to in-
spect the increment and adapt the product backlog if
needed. During the sprint review, the Scrum team and
doctors (two or three doctors who have different spe-
cialities), and members of the study group collaborate
about what was done in the sprint.
For the first six months, the sprint review should
be done twice a month. After that, the frequency can
be decreased to once a month. The meeting can take
between 15 minutes and 1 hour.
In addition to the elements defined in the Scrum
guide, the sprint review includes (1) the team prob-
lems to understand field types and relations; (2) doc-
tors’ comments and validation about cognitive work-
load; (3) the steps to do by participating doctors and
(4) discussion of technical issues, system interoper-
ability, privacy, confidentiality and lack of health in-
formation data standards.
5.3.5 Sprint Retrospective
The purpose of the meeting is to improve the process
for the next sprint. The entire Scrum team partici-
pates in the meeting. The retrospective takes place
just after the sprint review, and the speakers who have
attended can remain for the retrospective as observers
(Schwaber and Sutherland, 2001). The retrospective
sprint clarifies the points of interference with the doc-
An Adaptive Scrum Model for Developing Disease Registries
489

tors; a sharp intervention by them to verify the under-
standing may be planned for the next sprint.
5.4 Scrum Artifacts
Scrum artifacts represent information that the Scrum
team needs to insure inspection and adaptation. They
help the team to understand the product under devel-
opment, the activities done, and the activities being
planned in the project. Scrum defines the following
artifacts: product backlog, sprint backlog, increment
and burn-down chart. For a disease registry, the mod-
ification proposal is limited to the three first artifacts
(Schwaber and Sutherland, 2001).
5.4.1 Product Backlog
The Scrum product backlog is a prioritized feature
list, containing short descriptions of the functionality
desired for the product. In a disease registry project,
the product backlog includes:
• Disease form management operations: insert,
modify, list and delete. The deletion must be
logic. A module for logging the various actions
carried out by the user must be set up. Data veri-
fication algorithms may be required.
• User management operations: insert, modify, list
and delete. A group right must be clearly defined
to access data and to consult statistics. In the gen-
eral case, the doctor should consult and modify
only forms that (s)he has introduced.
• Data mining includes histogram presentations, pie
chart, or curves for:
– enumerating parameters (example: consan-
guinity)
– numerical parameters (example: weight), with
a possibility of zooming on particular zones
• Security requirements: who can do what?
5.4.2 Sprint Backlog
The sprint backlog is created during the sprint plan-
ning event, which is the first event in a sprint. A criti-
cal point in a disease registry project is the establish-
ment of the detailed plan for delivery of the items and
realization of the sprint goal during the sprint. Some
distributions may occur due to insufficient explana-
tions of requirements by the doctor for mathematical
or logical relations between fields. This detailed plan
will continue to be updated during the sprint.
5.4.3 Increment
An increment represents items made during the cur-
rent sprint and those that preceded it. At the end of a
sprint, the tasks included in the new increment must
be performed. This means it must be finished (i.e.,
developed and tested) and meet the Scrum teams def-
inition of done. In a disease registry, a task is done
when it is validated by at least one doctor.
6 APPLICATIONS
Three disease registries were developed based on the
proposed approach: The Tunisian Fanconi Anemia
Registry (TFAR), The Tunisian Non-Hodgkin Lym-
phoma Registry (NHLR) and the Tunisian Gaucher
Disease Registry (TGDR).
The TFAR was developed within one year. The
disease form has more than 200 fields. Doctors from
5 medical areas participated: hematology, oncology,
biology, pediatrics, cytogenetics. They belong to 10
hospitals and institutes. The project was developed by
8 members: 5 developers, 1 statistician, 1 human ma-
chine interface designer and 1 product owner. Scrum
daily meetings included one doctor and usually took
about 1 hour. The first backlog sprints contain expla-
nations of data fields and relations between them.
The NHLR was developed, in cooperation with
MDSoft
1
, during three years. The statistics module
is still in the works. The disease form includes more
than 1000 fields. Doctors from 2 medical areas par-
ticipated: hematology and oncology. They belong to
6 hospitals and institutes. The project was developed
by 7 members: 4 developers, 1 statistician, 1 human
machine interface designer and 1 product owner. The
duration of Scrum daily meetings is about a half hour,
with the participation of one doctor. A particular dif-
ficulty was experienced during Scrum planning. The
disease form is divided into sections. Each section
contains several input fields. During the merging op-
eration of the source code of the different sections,
we have encountered difficulties especially in the case
where there are relationships between the fields that
they contain. The sprint retrospective was compli-
cated due to the new requests raised by the doctors
after each trial of the product.
The second edition of the TGDR was established
in November 2016 in collaboration with the MD-
SOFT team. The disease form includes more than
250 fields. Doctors from 5 medical areas participate:
hematology, pediatrics, internal medicine, rheumatol-
ogy and gastroenterology. They belong to 6 hospitals
1
http://www.mdsoft-int.com
HEALTHINF 2017 - 10th International Conference on Health Informatics
490

and institutes. The project was developed by 6 mem-
bers. The product backlog has been updated several
times due to the instability of both medical and devel-
oper teams.
During these projects, our new methodology has
improved the following indicators: dedication (ad-
ditional sponteneous working hours reached up to
80%), focus (90% of the tasks were completed within
deadlines), openness (the rate of misunderstanding
between team members was less than 5%), audac-
ity (the team did not hesitate to name things, to ask
questions and to propose new solutions), and continu-
ity (despite major changes in team composition, the
projects did not have any delays in delivery). More-
over, the use of our methodology has reduced the risk
of failure by 95% in the case of TFAR.
7 CONCLUSION
In this paper, we have presented a new methodol-
ogy based on Scrum for disease registry development.
Several actions have been proposed to improve team
performance: (a) minimize the time of different iter-
ations, (b) facilitate code retrieval in the majority of
iterations, (c) clarify the descriptions and interactions
between different fields, (d) maximize collaboration
between the different teams and specialists involved,
namely doctors, computer scientists and statisticians.
Our methodology has been applied to the Tunisian
Fanconi Anemia Registry, the Tunisian Non-Hodgkin
Lymphoma Registry and the Tunisian Gaucher Dis-
ease Registry. The developed registries are currently
used in several hospitals in Tunisia.
In the future, we plan to define an effective
methodology for managing source code and deliver-
ables to improve team profitability.
ACKNOWLEDGEMENTS
This work is supported by the German Academic
Exchange Service (DAAD) (Transformation Partner-
ship: Theralytics Project).
REFERENCES
Beck, K., Beedle, M., Bennekum, A., Alistair, C., Cun-
ningham, W., Fowler, M., Grenning, J., Highsmith, J.,
Hunt, A., Jeffries, R., Kern, J., Marick, B., Martin, R.,
Mellor, S., Schwaber, K., Sutherland, J., and Thomas,
D. (2001). The agile manifesto. Agile Alliance, 2001.
Bourgeois, D. (2016). Information Systems for Business
and Beyond. Textbook Equity Edition.
Carroll, N., Ellis, J., Luckett, C., and Raebel, M. (2007).
Improving the validity of determining medication ad-
herence from electronic health record medications or-
ders. J. Am. Med. Inform. Assoc., 18(5):717–720.
Hammami, R., Bellaaj, H., and Kacem, A. (2014). In-
teroperability for medical information systems: an
overview. Health and Technology, 4(3):261–272.
Hummel, J. (2000). Building a computerized disease reg-
istry for chronic illness management of diabetes. Clin-
ical Diabetes, 18(5).
ISO 13119 (2012). Health informatics – clinical knowledge
resources – metadata. Standard, International Organi-
zation for Standardization.
ISO 13606-1 (2008). Health informatics – electronic health
record communication – part 1: Reference model.
Standard, International Organization for Standardiza-
tion.
ISO/TR 11633-1 (2009). Health informatics – information
security management for remote maintenance of med-
ical devices and medical information systems – part
1: Requirements and risk analysis. Standard, Interna-
tional Organization for Standardization.
ISO/TS 14441 (2013). Health informatics – security and
privacy requirements of EHR systems for use in con-
formity assessment. Standard, International Organiza-
tion for Standardization.
McCulloch, D., Price, M., Hindmarsh, M., and Wagner, E.
(1998). A population-based approach to diabetes man-
agement in a primary care setting: early results and
lessons learned. Effect. Clin. Pract., 1(1):12–22.
Schwaber, K. and Sutherland, J. (2001). The Scrum guide.
Staroselsky, M., Volk, L., Tsurikova, R., Newmark, L., Lip-
pincott, M., Litvak, I., Kittler, A., Wang, T., Wald,
J., and Bates, D. (2008). An effort to improve elec-
tronic health record medication list accuracy between
visits: patients’ and physicians’ response. Int. J. Med.
Inform., 77(3):153–160.
Sverrisdottir, H., Ingason, H., and Jonasson, H. (2014). The
role of the product owner in Scrum - comparison be-
tween theory & practice. Procedia - Social & Behav-
ioral Sciences, 19(3):257–267.
Vickoff, J. (2000). RAD, le developpement rapide
d’applications. RAD.fr.
An Adaptive Scrum Model for Developing Disease Registries
491
