
Fluorine Phosphate Glasses Doped with Cadmium Sulfide and
Selenide Quantum Dots with High Quantum Efficiency at
Room-temperature
E. V. Kolobkova
1,2
, Zh. Lipatova
1
, M. S. Kuznetsova
3
and N. Nikonorov
1
1
Department of Optical Informatics Technologies and Materials, ITMO University, Saint-Petersburg, Russia
2
St. Petersburg State Institute of Technology, Technical University, St. Petersburg, Russia
3
Saint-Petersburg State University, St. Petersburg, Russia
Keywords: Quantum Dots, Fluorine-phosphate Glass, Luminescence, Absolute Quantum Yield.
Abstract: The results of the study of the luminescent properties of the CdS(Se) quantum dots (QDs) with the mean
size of 2-4 nm synthesized in the fluorine phosphate glass are discussed. The changes of the
photoluminescence absolute quantum yield (PL AQY) magnitude of the CdS(Se) QDs with various mean
sizes induced by the heat treatment are studied. It was found that the PL AQY of the CdSe QDs increases
monotonically to a maximum and then fells down. PL AQY magnitudes for glasses doped with CdS QDs
demonstrate weak dependence on the size. It was found that CdS(Se) QDs represents a series of excellent
emitters in the 600-750 nm spectral region. PL AQY in the glasses can reach 50-65%, which is equal to the
value in the colloidal nanocrystals and higher than it was reported earlier for the silicate glasses. The glass
matrix protects the QDs from external influence and their optical properties remain unchanged for a long
time.
1 INTRODUCTION
QDs are a type of nanomaterials with good
fluorescent properties. The size-dependent emission
is probably the most attractive property of
semiconductor nanocrystals.
Among them, CdS and CdSe QDs are one of the
most promising materials because QDs have bright
luminescence in the visible range of the optical
spectrum. For example, CdSe QDs have shown
potential as superior biological labels (Han, M. et
al., 2001, Bruchez, M. et al., 1998 and Chan, W. C.
W., Nie, S. M., 1998), laser sources (Artemyev, M.
et al., 2001, Klimov, V. I.et al., 2000) and tunnel
diodes (Sundar, V. C. et al., 2000, Schlamp, M.C.,
Peng, X., Alivisatos,A.P., 1997).
Comparing with conventional fl uorescent dyes
CdS(Se) QDs have a wide continuously distributed
excitation spectra, not only with symmetrical
distributed narrow emission spectra, but also many
other excellent properties such as adjustable color,
excellent photochemical stability and high threshold
of light bleaching (Qu, L., Peng, X. 2002).
However, colloidaly synthesized bare quantum
dots, including CdSe(S) QDs, usually have surface
defects, which diminish photoluminescence (PL)
absolute quantum yield. The best PL AQY reported
for the as-prepared nanocrystals at room temperature
is around 20% in the wavelength range between 520
and 600 nm and is about a few percent or lower in
the wavelength range above 600 nm and below 520
nm (Sundar, V. C. et al., 2000). In general, a low PL
AQY is considered as a result of the surface states
located in the bandgap of the nanocrystals, which act
as trapping states for the photogenerated charges.
These surface trapping states are originated from the
dangling bonds of some of the surface atoms (Fu,
H.; Zunger, A. 1997, Xu, K. M. et al., 2010 and Kim
J, M. et al., 2012). That`s why it is essential to
control the QDs surfaces to reduce the surface
defects by passivating the surface of QDs (Talapin,
D.V. et al., 2001). The core/shell structures solve
optical problems, such as low PL AQY, and improve
the stability of QDs. In (Talapin, D.V. et al., 2001)
was shown that the room-temperature quantum
efficiency of the band edge luminescence of CdSe
QDs can be improved to 40-60% by surface
328
Kolobkova E., Lipatova Z., Kuznetsova M. and Nikonorov N.
Fluorine Phosphate Glasses Doped with Cadmium Sulfide and Selenide Quantum Dots with High Quantum Efficiency at Room-temperature.
DOI: 10.5220/0006266203280333
In Proceedings of the 5th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2017), pages 328-333
ISBN: 978-989-758-223-3
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

passivation with inorganic (ZnS) or organic
(alkylamines) shells. Annealing of the samples in
different environments (oxygen, hydrogen, and air)
seems to reduce the PL emission, due to the
activation of non-radiative defect states.
Due to the failure of orange-red emitting
materials in general, efforts in colloidal CdS/CdSe
QDs formation were mainly concentrated on the
wavelength region between 600 nm (orange) and
650 nm (red). Nevertheless, the stability and the
reproducibility of the PL AQY are both not
predictable. With some inorganic and organic
surface passivation after the synthesis, the PL QY of
the colloidal CdSe nanocrystals is boosted more than
50% in the range 520-600 nm, but the efficiency for
the orange-red color window is still low. Especially
for red (around 650 nm), the PL QY of the
nanocrystals in solution was nearly zero (Qu, L.,
Peng, X. 2002).
Semiconductor nanoparticles CdS and CdSe
dispersed in a silicate glass matrix are attracting
much attention (Borreli, N. F. et al., 1987, Su, Z., et
al., 1996 and Xu, K. et al., 2010). The possibility of
QDs formation in the optical material creates
significant benefits for their application. Currently,
optical transparent glasses doped with nanocrystals
are of the great interest for the modern element base
of photonics. These materials combine the best
properties of nanocrystals and glasses (possibilities
of pressing and molding, spattering, pulling optical
fibers). In addition, the glass matrix protects the
QDs from external influence. In the silicate glasses,
the PL spectra consist of two bands: a less intense
high-energy band, and a lower energy broader band.
First band occurs at a wavelength 10-20 nm higher
than the absorption edge and is due to direct
electron-hole recombination. This peak shifts to the
higher wavelength with increasing particle size.
Second band is due to surface defects and occurs at
800-900 nm spectral region. PL AQY is less than
30% for CdS(Se) QDs in the silicate glasses and
decreases as a size of QD increases (Borreli, N.F. et
al., 1987).
In this study, we used a fluorine phosphate (FP)
glass featuring with a number of advantages
compared to conventional silicate glasses, including
low temperature synthesis, possibility for a wide
range adjustment of the formed quantum dots
concentration, low temperature and time of heat
treatment and higher spatial distribution
homogeneity. Two-stage heat treatment afforded
quantum dots with narrow size distribution. The CdS
and CdSe QDs dispersed in a fluorine phosphate
glasses are attracting much attention as nonlinear
materials (Vaynberg, B. et al., 1996 and Lipovskii,
A.A. et al., 1999), but information about
luminescent properties is not available.
In this study, we represent the luminescent
properties of a fluorine phosphate glass doped with
CdS and CdSe QDs.
2 EXPERIMENTAL
In order to investigate the effect of the QDs sizes on
the PL properties, the fluorine
-phosphate (FP)
glasses 0.25Na
2
O-0.5P
2
O
5
-0.05ZnF
2
-0.1Ga
2
O
3
-
0.05AlF
3
-0.05NaF (mol. %) doped with CdS or
CdSe were synthesized. The glass synthesis was
conducted in an electric furnace at 1050
o
C in the Ar-
atmosphere using the closed glassy carbon crucibles.
After quenching, the glasses were annealed at 320 C
for 1 hour to release thermal stress, cut into pieces of
10.0 x 10.0 mm, and then were optically polished.
Planar polished samples 0.4-1.0 mm thick were
prepared for further investigation. The glass
transition temperature measured with STA 449 F1
Jupiter (Netzsch) differential scanning calorimeter
was found to be 390±3 C. Samples were heat treated
using a muffle furnace (Nabertherm) with program
temperature control to induce formation of CdS(Se)
QDs at 410
o
C. The optical density spectra of the
studied FP glass samples were recorded using a
double-beam spectrophotometer Lambda 650
.
(Perkin Elmer) in the 1.5-5 eV spectral region with
0.1 nm resolution. For registration of the emission
spectra excited at λ =405 nm (3.06 eV) was used an
EPP2000-UVN-SR (Stellar Net) fiber spectrometer.
The luminescence was excited by semiconductor
lasers (=405 nm). All measurements were
performed at room temperature. Absolute quantum
yield measurements were carried out inside the
integrated sphere with Photonic Multichannel
Analyzer (PMA-12, Hamamatsu) at room
temperature. The measurement error for the absolute
quantum yield (AQY) was ±1%.
3 RESULTS AND DISCUSSIONS
3.1 Glasses Doped with Cadmium
Selenide Quantum Dots
The emission properties of semiconductor
nanocrystals can be characterized by three
fundamental parameters, which are the brightness,
the emission color, and the stabilityof the emission.
Fluorine Phosphate Glasses Doped with Cadmium Sulfide and Selenide Quantum Dots with High Quantum Efficiency at Room-temperature
329
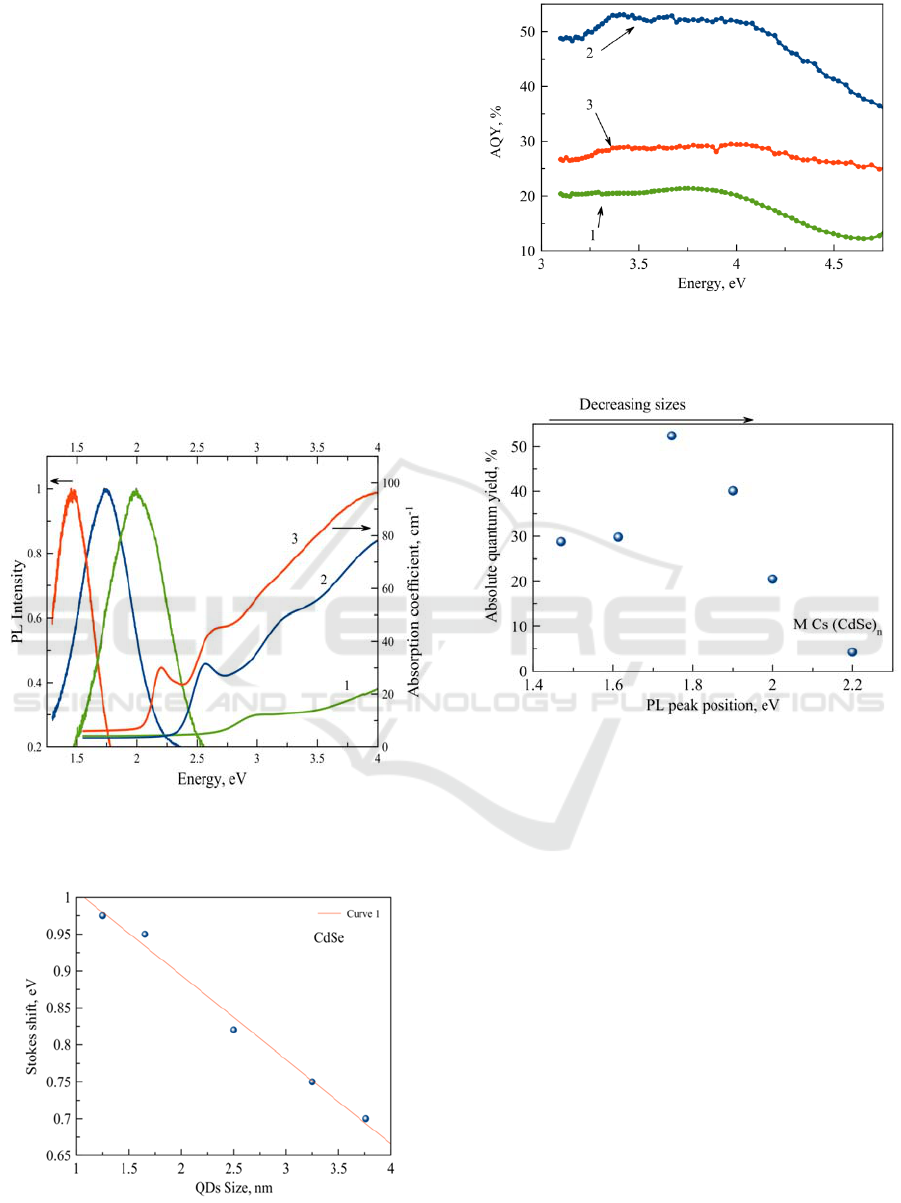
A samples of the glass containing CdSe QDs
were prepared by heat treatment of a 0.25Na
2
O-
0.5P
2
O
5
-0.05ZnF
2
-0.1Ga
2
O
3
-0.02PbF
2
-0.08AlF
3
glass doped with 0.6 mol. % CdSe at T=410
o
C
during 20-60 min.
Due to quantum size effects, the band gap of
CdSe QDs increases from 2.2 eV to 3.0 eV, as the
size of the nanocrystals decreases from 4.0 nm to 2.0
nm. The emission color of the PL of the nanocrystals
shifts continuously from red (centered at 730 nm) to
orange (centered at 630 nm) as size of QDs decrease
(Fig1). QDs sizes were defined using data (Norris,
D. J. and Bawendi M. G. 1996).
In the PL spectra, the broad band with a large
Stokes shift is dominant, and the band-edge PL is
negligibly weak. The emission spectrum of samples
is dominated by "deep trap" emission, strongly red
shifted from the band edge (Fig2).
Figure 1: Absorption and luminescence spectra of the
glass doped with CdSe QDs with a sizes 2.0 nm (1), 3.0
nm (2), 4.0 nm (3). The excitation energy is 3.06 eV.
Figure 2: Dependence of the Stokes shift on the QDs size.
Figure 3: Dependence of the PL AQY for glasses doped
with CdSe QDs with sizes 2.0 nm (1), 3.0 nm(2), 4.0 nm
(3) on the excitation energy.
Figure 4: Dependence of the PL AQY on the CdSe QDs
sizes.
Fig. 3 demonstrates dependence of the AQY
magnitudes for glasses doped with CdSe QDs. The
QDs concentration in the glasses 2 and 3 is equal
(Fig.1), but AQY of the QD with size 3 nm is in two
times higher. The emission color of the PL of the
QDs with size 3 nm is red with λ
max
=700 nm
The PL AQY magnitudes for glasses doped with
CdSe QDs demonstrate nonlinear dependence on the
size (Fig. 4).The PL AQY of the QDs increases
monotonically to a maximum and then fells down to
30 % (Fig. 4). For convenience, the position with the
maximum PL AQY is called the bright point as in
(Qu, L., Peng, X. 2002). The bright point for CdSe
QDs in FP glass is observed for QDs with size 3.0
nm.
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
330
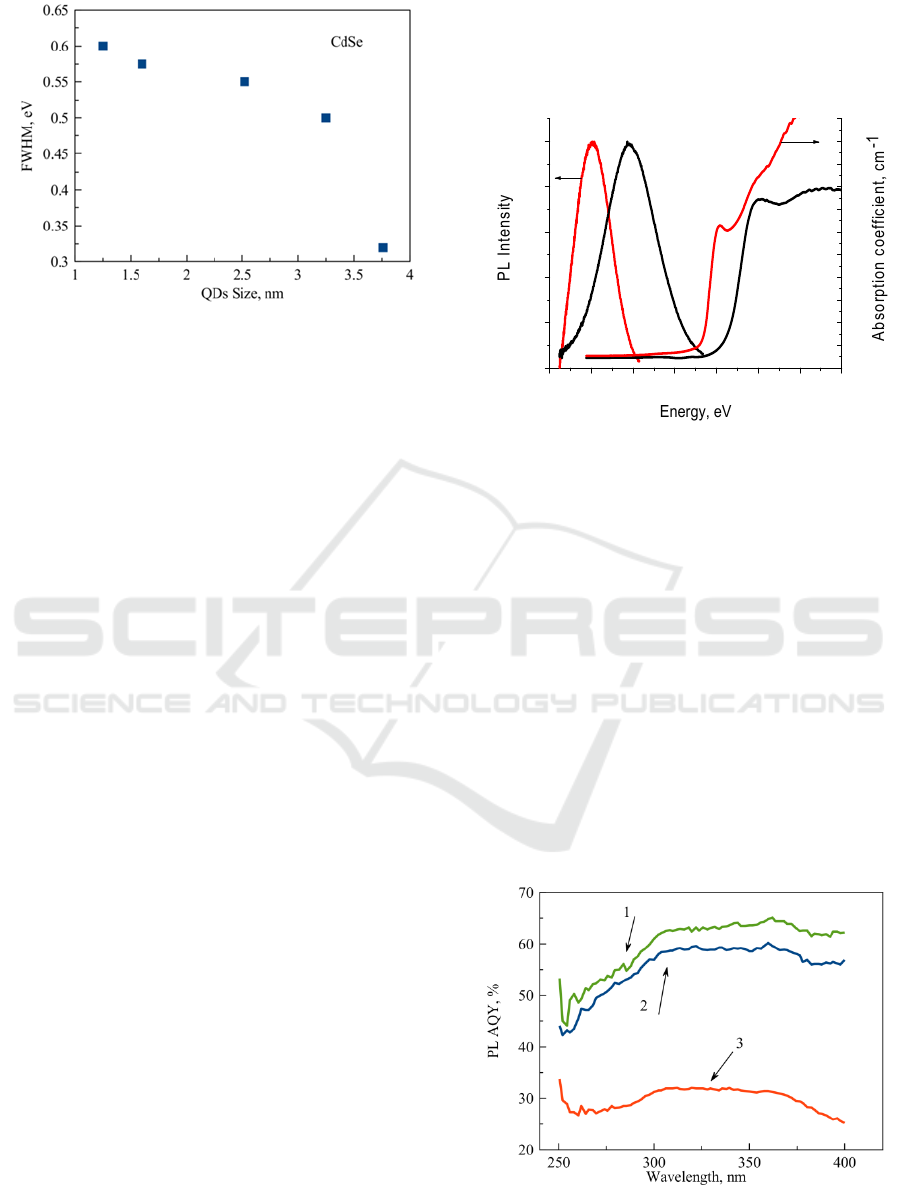
Figure 5: Dependence of the PL FWHM magnitudes for
glasses doped with CdSe QDs with different sizes.
The typical full width at half-maximum
(FWHM) of the PL peak of the CdSe QDs ensemble
at room temperature in FP glass, around 600-300
meV, is noticeably broader than that observed for
colloidal QDs (Qu, L., Peng, X. 2002).
FWHM
magnitudes decrease as QDs sizes increase (Fig 5).
The values of the PL AQY of the samples 1, 2
and 3 were measured several times during the year.
The results of the measurements coincided, which
confirmed the stability of luminescent
characteristics.
3.2 Glasses Doped with Cadmium
Sulfide Quantum Dots
As it was shown in (Lipatova, Zh.O., Kolobkova
E.V., Aseev,V.A. 2015) heat treatment has a
significant impact on properties of glasses doped
with CdS QDs. Absorption peaks due to confined
excitons are clearly observed in the higher-energy
region compared with the band-gap energy of 2.5 eV
in a CdS bulk crystal. These results evidently
indicate the formation of CdS QDs. With increasing
of the heat treatment duration, the exciton-
absorption peak shifts to a lower-energy side. Based
on a theory of the quantum size effect in spherical
QDs (Martin J.L., Rivera R., Cruz S.A. 1998) the
mean radii of prepared CdS QDs are estimated to be
2.3 and 3.5 nm. The observation of the clear
absorption peaks indicates that the size-distribution
width of the CdS QDs is rather small (Fig 6).
Fig. 6 clearly shows effect of the heat treatment
on the absorption and emission spectra of FP glasses
doped with CdS QDs with sizes 2.3 and 3.5 nm. In
PL spectra, the broad PL band with a large Stokes
shift (1.2 eV) is dominant, and the band-edge PL is
negligibly weak. The emission spectrum of samples
is dominated by "deep trap" emission, strongly red
shifted from the band edge (Fig.6).
Fig. 7 demonstrates concentration and
size
dependence of the PL AQY magnitudes for glasses
doped with CdS QDs.
1,2 1,6 2,0 2,4 2,8 3,2 3,6 4,0
0,0
0,2
0,4
0,6
0,8
1,0
1
2
1
2
0
10
20
30
40
50
60
70
80
90
100
110
Figure 6: Absorption and luminescence spectra of the
glasses doped with CdS QDs with a sizes 2.3 nm (1), 3.5
nm (2). The excitation energy is 3.06 eV.
Concentration of the QDs in the glass 3 is in two
times higher than in glass 2. Comparison of the PL
AQY of the two glasses doped with different QDs
concentration (Fig. 7, curves 3 and 1) demonstrates
PL concentration quenching as number of QDs
increases above concentration threshold (Fig. 7).
The PL AQY magnitudes for glasses doped with
CdS QDs with sizes 2.3 -3.5 nm demonstrate weak
dependence on the size (Fig. 7). The PL AQY of the
CdS QDs increases to 65 % for QDs with size 2.3
nm and then slowly fells down to 60 % for QDs with
size 3.5 nm (Fig. 7). The values of the PL AQY of
the samples 1, 2 and 3 were measured several times
during the year. The results of the measurements
coincided.
Figure 7: Dependence of PL AQY magnitudes for glasses
doped with CdS QDs with sizes 2.3 nm (1), 3.5 nm (2) and
2.5 nm (3), respectively, on the excitation wavelength.
Fluorine Phosphate Glasses Doped with Cadmium Sulfide and Selenide Quantum Dots with High Quantum Efficiency at Room-temperature
331

Absolute quantum yield allows estimating
efficiency of converting UV light in the visible
range that is why it is an important parameter for
industrial applications of glasses doped with
CdS(Se) QDs as luminescence down shifting
material or phosphor.
4 CONCLUSIONS
The CdS(Se) nanocrystals synthesized in the
fluorine phosphate glass represents a series of
excellent emitters in the orange-red spectral region
(600-750 nm) in terms of their PL AQY and the
FWHM of the PL spectra, and they show the
stability of the emission for a long time.
The photoluminescence quantum yield of CdSe
QDs rises monotonically to a maximum value and
then decreases gradually with QDs size increase.
Such a maximum (a PL “bright point”) is in 650-750
nm spectral range.
The PL AQY magnitudes for glasses doped with
CdS QDs with sizes 2.3 -3.5 nm demonstrate weak
dependence on the size.
We suggest that origin of these dependences is
the difference in the interaction mechanisms
between CdSe, CdS quantum dots and glass-
network.
Experimental results suggest that the existence of
the PL bright point is general phenomenon of CdSe
QDs and likely is signature of an optimal surface
structure reconstruction of the nanocrystals grown in
a liquid (Qu, L., Peng, X. 2002) or in glass. Absolute
quantum yield magnitude of luminescence glasses
doped with CdS(Se) QDs can reach 50-65%, which
is in two times higher than it was reported earlier in
the silicate glasses. It opens up new prospects for
using such materials as phosphors for white LEDs
and down-convertors for solar cells.
ACKNOWLEDGEMENTS
Research was funded by Russian Science
Foundation (Agreement #14-23-00136).
REFERENCES
Han, M. et al., 2001. Quantum-dot-tagged microbeads for
multiplexed optical coding of biomolecules. Nat.
Biotechnol. 19. pp 631-635.
Bruchez, M. et al., 1998. Semiconductor nanocrystals as
fluorescent biological labels. Science. 281. pp 2013-
2016.
Chan, W.C.W., Nie, S. M. 1998. Quantum dot
bioconjugates for ultrasensitive nonisotopic detection.
Science, 281, pp 2016-2018.
Artemyev, M. et al.,2001. Light trapped in a photonic dot:
Microspheres act as a cavity for quantum dot
emission. Nano Lett., 1. Pp 309-314.
Klimov, V. I. et al., 2000. Optical Gain and Stimulated
Emission in Nanocrystal.Science, 290,pp 314-317.
Sundar, V. C. et al.,2000, Full color emission from II-VI
semiconductor quantum dot-polymer composites
Adv.Mater. 12. Pp 1311-1312; Novel light emitting
devices using cadmium selenide nanocrystals.
Abstracts of papers of the American chemical
society.220. ppU206-U206.
Schlamp, M.C., Peng, X., Alivisatos, A.P., 1997.
Improved efficiencies in light emitting diodes made
with CdSe(CdS) core/shell type nanocrystals and a
semiconducting polymer. J. Appl. Phys. 82. Pp 5837–
5842.
Qu, L., Peng, X. 2002. Control of Photoluminescence
Properties of CdSe Nanocrystals in Growth. J. Am.
Chem. Soc. 124(9).pp 249-255.
Fu, H.; Zunger, A. 1997. InP quantum dots: Electronic
structure, surface effects, and the redshifted emission.
Phys. Rev. B: Condens. Matter, 56, pp 1496-1508.
Talapin D.V., et al.,2001. Highly luminescent
monodisperse CdSe and CdSe/ZnS nanocrystals
synthesized in a hexadecylamine-trioctylphosphine
oxide-trioctylphospine mixture, Nano Lett. 14. pp
207–211.
Wang, X et al.,2003. Surface-Related Emission in Highly
Luminescent CdSe Quantum Dots. Nano Lett., 3(8) pp
1103-1106.
Borreli, N.F. et al.,1987. Quantum confinement effects of
semiconducting microcrystallites in glass, J. Appl.
Phys. 61.pp 5399–5409.
Su, Z.et al., 1996. Selenium molecules and their possible
role in deep emission from glasses doped with
selenide nanocrystals, J. Appl. Phys. 80.Pp 1054–105.
Vaynberg, B. et al., 1996. High optical nonlinearity of
CdS
x
Se
1-x
microcrystals in fluorine-phosphate glass
Optics communications. 132 (3-4). Pp 307-310.
Lipovskii, AA. et al., 1999. Formation and growth of
semiconductor nanocrystals in phosphate glass matrix.
Journal of the European ceramic society.19 (6-7). Pp
865-869.
Xu, K.M. et al., 2010. Optical properties of CdSe quantum
dots in silicate glasses. J Non-Crystalline
Solids.356.Pp 2299–2301.
Martin, J.L., Rivera, R., Cruz, S.A. 1998. Confinement of
excitons in spherical quantum dots. J. Phys.:
Condence Matter. 10. Pp1349–1361.
Kim, J, M. et al., 2012. Photoluminescence enhancement
in CdS quantum dots by thermal annealing. Nanoscale
Research Letters. 7. Pp 482–489.
Lipatova, Zh.O., Kolobkova, E.V., Aseev,V.A. 2015.
Kinetics and luminescence of cadmium sulfide
quantum dots in fluorine-phosphate glasses, Opt.
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
332

Spectrosc. 119(I2), pp 229–233.
Norris, D. J. and Bawendi M. G. 1996. Measurement and
assignment of the size-dependent optical spectrum in
CdSe quantum dots. Physical Rev. B. 53, (24). pp
16338-16446.
Norris, D. J. and Bawendi, M. G. 1995. Structure in the
lowest absorption feature of CdSe quantum dots. .
Chem. Phys., 103(130). pp5260-5268.
Fluorine Phosphate Glasses Doped with Cadmium Sulfide and Selenide Quantum Dots with High Quantum Efficiency at Room-temperature
333
