
PMMA/MEH-PPV Photoluminescent Polymer Blend as a Long Time
Exposure Blue-light Dosimeter
José Roberto Tozoni, Alexandre Marletta,
Adryelle do Nascimento Arantes and Luana Rodrigues de Oliveira
Institute of Physics, Federal University of Uberlandia, P. O. Box 593, Uberlandia, 38400-902, Minas Gerais, Brazil
Keywords: Host/Guest, Polymer Blend, Poly(Methyl Methacrylate), Poly[2-Methoxy-5-(2-Ethylhexyloxy)-1,4-
Phenylenevinylene], Photoemission, Long Time Exposure Blue-light Dosimeter.
Abstract: In the present paper the photoemission intensity versus excitation exposure time of host/guest
photoluminescent polymer blend has been investigated. The polymer blend was composed by poly(methyl
methacrylate) (PMMA) as a host, and Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-
PPV) as a guest. The photoluminescent blend was characterized by optical absorbance and steady-state
photoluminescence spectroscopy. The PMMA/MEH-PPV blend film presented high homogeneity and high
photoemission intensity. Moreover, the PMMA/MEH-PPV blend film photodegradation in function of
sample exposure time to the blue-light excitation curve presented long biexponential time decay. These
results suggest that the PMMA/MEH-PPV blend film could be used as a long time exposure blue-light
dosimeter.
1 INTRODUCTION
Conjugated polymers have received great scientific
and technological attention due to its applications in
the areas of light-emitting, photovoltaic and sensors
devices (Yu, 1996, Hide, 1997, Leclerk, 2001, Silva,
2011, Ferreira, 2014). The biggest challenges in the
development of polymer conjugated devices are
increase the light emission efficiency and the life-
time (Leclerk, 2001, Yan, 1994, Atreya, 1999, Yu,
2000, Jorgensen, 2008, Palacios-Lindon, 2013). In
this system the macromolecular association is,
generally, an undesired process, which decreases the
efficiency of the light emission (Huser, 2001,
Tozoni, 2009, Spano, 2014, Chou, 2005, Mirzov,
2006, Lin, 2010).
Moreover, the conjugated polymer
photodegradation process cause changes in the
polymers structures and physical properties and has
lethal effects on efficiency of the devices (Silva,
2011, Ferreira, 2014, Yan, 1994, Atreya, 1999, Yu,
2000, Jorgensen, 2008, Palacios-Lindon, 2013). In
contrast, the alteration in the structural,
photoemission and the optical properties of solutions
and films of conjugated polymers, produced due the
photodegradation process, has been used in the
development of ionizing and non-ionizing radiation
dosimeters (Silva, 2011, Ferreira, 2014).
The photodegradation process is oxygen
dependent and has two possible pathways involving
the generation of either singlet oxygen or superoxide
radical anions (Atreya, 1999, Yu, 2000, Jorgensen,
2008, Palacios-Lindon, 2013, Soon, 2013).
Furthermore, dosimeters based in conjugated
polymers solutions, due the use of organic solvent,
are not safe for medical utilization. In addition, due
the macromolecular aggregation the films of
conjugated polymers have low photoemission
intensity and it is very difficult to obtain a structured
film using a small amount of conjugated polymers.
Other problem to use the conjugated polymers as
a long time irradiation exposure dosimeter is the fast
exponential time decay of the photoemission
intensity (Lee, 2011).
In a preceding paper, we have shown the de-
aggregation of the poly(9,9-di-hexylfluorenediyl
divinylene-alt-1,4-phenylenevinylene) (LaPPS16),
an electroluminescent polymer which shows a high
tendency to π-stacking aggregation, through the
formation of photoluminescent polymers blendes of
the LaPPS16 with several members of a series of
poly(n-alkyl methacrylate)s (Tozoni, 2009). The
PnMA/LaPPS16 blends present high mechanical
Tozoni J., Marletta A., Arantes A. and de Oliveira L.
PMMA/MEH-PPV Photoluminescent Polymer Blend as a Long Time Exposure Blue-light Dosimeter.
DOI: 10.5220/0006261403170322
In Proceedings of the 5th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2017), pages 317-322
ISBN: 978-989-758-223-3
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
317

properties and the photoluminescence spectra
presented high intensity and efficiency emission
(Tozoni, 2009).
Based on the above settings; in the fact of the
photodegradation process is oxygen dependent that
is dependent of the oxygen diffusion coefficient of
the polymers (Yu, 2000, Jorgensen, 2008, Shoee,
2015, Rothberg, 1996), in the fact that the PMMA
can works as a oxygen barrier (Yu, 2000, Jorgensen,
2008) and with the purpose to develop long time
blue-light dosimeters with greater light emission
efficiency, good mechanical properties and safer for
medical applications, in this paper was studied the
photodegradation of a photoluminescent polymer
blend composed by poly(methyl methacrylate)
(PMMA) as a host, and Poly[2-methoxy-5-(2-
ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV)
as a guest. The results show that the PMMA/MEH-
PPV photoluminescent blend form a well structured
film with high homogeneity and high photoemission
intensity efficiency. Moreover, the PMMA/MEH-
PPV blend photodegradation curve presented long
biexponential time decay. These results suggest that
the PMMA/MEH-PPV blend could be used as a long
time blue-light dosimeter.
2 MATERIALS AND METHODS
The poly(methyl methacrylate) (Mw=350,000) was
purchased from Scientific Polymer Products Inc. and
was used as received. Poly[2-methoxy-5-(2-
ethylhexyloxy)-1,4-phenylene vinylene] (MEH-
PPV) and the chloroform was purchased from
Sigma-Aldrich, and was used as received too.
Solutions of PMMA/chloroform (262.5mg/15mL)
and PMMA/MEH-PPV/ chloroform
(262.5mg/0.216mg/15mL) and were prepared using
three sequential cycles of sonication (5 minutes) and
mechanically stirring (5 minutes) at 60
o
C. Then,
each solution were cast in one Petri dish at ambient
conditions and allowed to dry for a week, in the
dark. After the solvent evaporation the PMMA and
PMMA/MEH-PPV samples formed films with
thicknesses of about 100 m. Dried films of PMMA
and PMMA/MEH-PPV with dimensions of ~1.5x1.5
cm were separated for analyses.
AFM images were recorded using the Shimadzu
Scanning Probe Microscope (SPM-9600). Optical
absorption spectra were recorded using the
spectrophotometer FEMTO 800 XI. The steady-state
photoluminescence excitation (PLE) spectrum was
recorded on a Hitachi U-2001 spectrofluorometer.
The PMMA/MEH-PPV photoluminescence
emission spectra, over the entire band (500-800nm)
in function of exposure time to the blue-light
excitation and at ambient conditions, were obtained
by exciting the samples with a low-pressure mercury
vapour fluorescent lamp emitting light in the blue
part of the visible spectrum (Philips TL 20W/52
emission spectra over the entire band 400-540nm
with maxima at 450nm). This lamp is normally used
in incubators for the treatment of
Hyperbilirubinaemia in neonates. The
PMMA/MEH-PPV photoluminescence emission
spectra were acquired by Ocean Optics spectrometer
USB2000. The blend film was put at 38 cm apart the
lamp, the irradiance incident on the blend film
region was 5mW/cm
2
. Figure 1 shows the scheme of
the experimental setup.
Figure 1: Scheme of the experimental setup used for the
acquisition of the photoluminescence spectra versus time
of exposure to the blue-light excitation.
3 RESULTS AND DISCUSSION
Figure 2 shows the PMMA/MEH-PPV blend film
image after 45.50 hours of irradiation. Visually it is
observed that the sample presents great homogeneity
without presenting phase segregation.
Figure 2: PMMA/MEH-PPV blend image after 45.50
hours of irradiation exposure.
Moreover, due the reduction of absorption
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
318
(photobleaching in the central region), it is possible
to differentiate the irradiated region from the non-
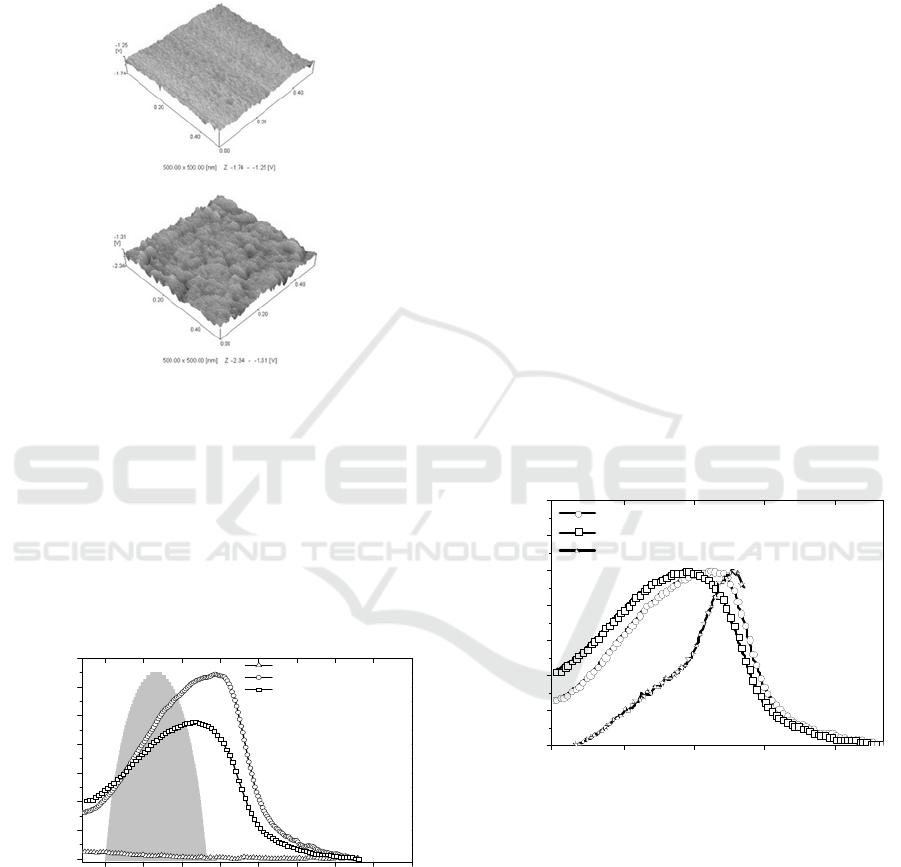
irradiated region. Figure 3 shows the PMMA/MEH-
PPV blend film AFM images of the regions non-
irradiated and after 45.50 hours of irradiation
exposure.
Non-irradiated
Irradiated
Figure 3: PMMA/MEH-PPV blend AFM images before
and after 45.50 hours of irradiation exposure.
The AFM images show that the photo-oxidation
changes the blends morphology favoring the
formation of nanostructures
increasing the roughness
of the blend surface. Figure 4 shows the optical
absorbance spectra of the PMMA and
PMMA/MEH-PPV blend film in the UV-Vis range
and the Lamp Emission region (gray region).
Figure 4: Absorbance spectra of the cast film of PMMA
(open triangle), the PMMA/MEH-PPV blend film before
irradiation exposure (open circle) and PMMA/MEH-PPV
blend film after 45.50 hours of irradiation exposure (open
square). The gray inset figure shows the Lamp Emission
region.
The absorbance spectrum of PMMA shows that the
PMMA/MEH-PPV absorbance was independent of
the PMMA matrix. Furthermore, the absorbance
spectra of PMMA/MEH-PPV blend film are
broader, with maxima at ~542 nm before irradiation,
and ~517 nm after 45.50 hours o of irradiation
exposure. Figure 5 shows the normalized PLE
spectrum of the PMMA/MEH-PPV before
irradiation at wavelength detection of 590nm
(
Det
=590nm) and the normalized absorbance spectra
of the PMMA/MEH-PPV before and after 45.50
hours of irradiation exposure. These results show
that the absorbance spectra line shapes were
dependent of the film exposure time to the
excitation. After 45.50 hours of irradiation exposure
the PMMA/MEH-PPV blend film absorbance
spectrum was broadened, presents a significant blue-
shift (~25nm) and intensity decrease. The optical
absorbance spectrum modifications in function of
the film exposure time are due the photo-oxidation
that promotes changes in the chemical structure of
the PMMA/MEH-PPV blend film. These results
show that the photo-oxidation changes the
distribution of effective conjugation length to shorter
chains and increase the microscopic disorder (Yan,
1994, Rothberg, 1996, Atreya, 1999). The PLE
spectrum presents two maxima, one around ~566nm
(maximum of efficiency) and other around ~480nm.
37 5 45 0 525 600 675
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
Absorbance ( 0.00 h)
Absorbance ( 45.50 h)
PLE (0. 0 0 h)
Normalized Intensity
Wavel ength (n m)
Figure 5: Normalized PLE spectrum of the PMMA/MEH-
PPV before irradiation exposure at wavelength detection
of 590nm (open star), the normalized absorbance spectra
of the PMMA/MEH-PPV blend film before irradiation
exposure (open circle) and after 45.50 hours of irradiation
exposure (open square).
The PLE spectrum maximum was red shifted
approximately 24 nm as compared to the optical
absorbance spectra. The difference between the
absorbance and PLE normalized spectra of the
PMMA/MEH-PPV blend (before irradiation)
suggests that, due the presence of aggregated
400 450 500 550 600 650 700 750 80
0.00
0.05
0.10
0.15
0.20
0.25
0.30
0.35
Lamp
Emission
Region
PMMA
PMMA/MEHPPV (0.00 h)
PMMA/MEHPPV (45.50 h)
Absorbance
Wavelength (nm)
PMMA/MEH-PPV Photoluminescent Polymer Blend as a Long Time Exposure Blue-light Dosimeter
319
species, the exciton diffusion is very efficiently.
Due to the fact that MEH-PPV photo-oxidation
results in the creation of carbonyl groups that
quench the photoluminescence intensity (Rothberg,
1996; Yu, 2000; Jorgensen, 2008), and in order to
quantify and correlate this effect with the film
exposure time to the lamp light excitation measures
of the photoluminescence spectra of the
PMMA/MEH-PPV blend film in function of the
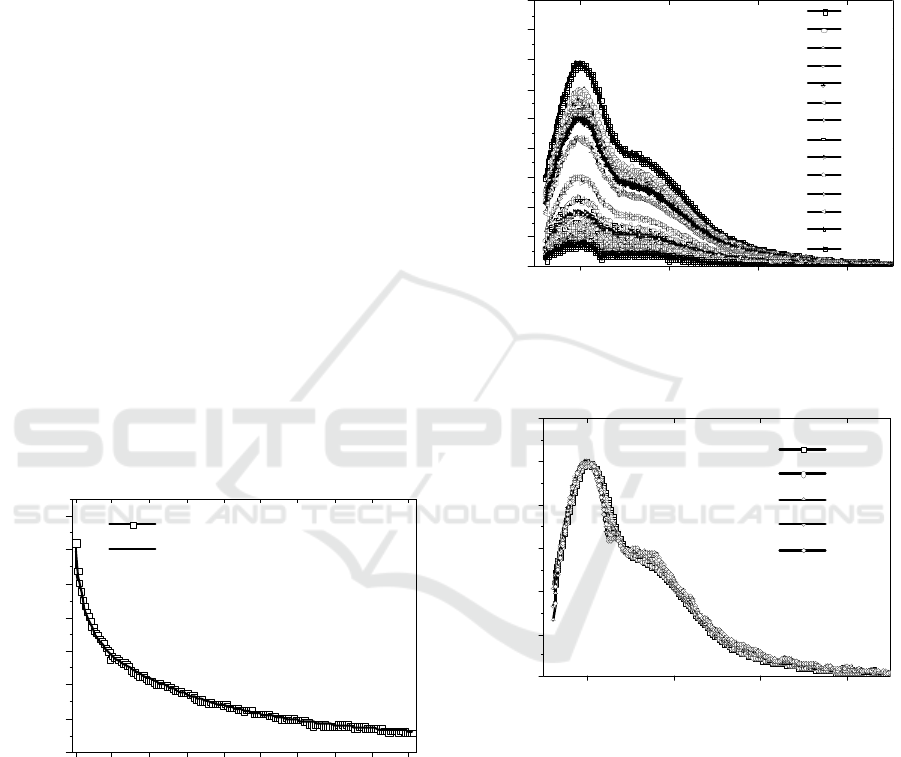
irradiation exposure time have been done. Figure 6
shows the PMMA/MEH-PPV blend film Integrated
PL intensity (over the entire band 580-800nm) in
function of sample time of exposure to the lamp
light excitation curve (0.00 to 45.50 hours in
intervals of 0.25 hours, open square) and the
biexponential fitting equation 1 (solid line).
I
t
=I
+I
e
/
+I
e
/
(1)
The curve present a bi-exponential time decay
behavior with a long time t
1
= 16.67 hours and a fast
time t
2
=1.40 hours). Since the MEH-PPV photo-
oxidation depends on the oxygen diffusion in to the
sample (Yu, 2000, Jorgensen, 2008, Shoee, 2015,
Rothberg, 1996) and the PMMA can works at a
oxygen barrier (Yu, 2000, Jorgensen, 2008),
probably the fast decay time t
2
was due the photo-
oxidation of the superficial MEH-PPV and the long
decay time t
1
was due the bulk MEH-PPV photo-
oxidation.
Figure 6: PMMA/MEH-PPV blend film Integrated PL
intensity in function of the irradiation exposure time (open
square) and the bi-exponential fitting curve (solid line).
Figure 7 shows some PMMA/MEH-PPV blend film
PL spectra in function of sample irradiation
exposure time. The PL spectra present two well
defined maxima, one around ~600nm (0-0
transition) and other around ~640nm (0-1 transition).
Figure 8 shows some PMMA/MEH-PPV blend
film PL normalized spectra in function of sample
irradiation exposure time. No significant changes in
the MEH-PPV PL spectra line shapes were
observed, probably there was no formation of
aggregates and the PL intensity decrease is basically
due the carbonyl formations that work as a PL and
excitons quenchers (Atreya, 1999; Yu, 2000,
Jorgensen, 2008; Rothberg, 1996).
Figure 7: Various PMMA/MEH-PPV blend film PL
spectra in function of sample irradiation exposure time.
Figure 8: Some PMMA/MEH-PPV blend film PL
normalized spectra in function of sample irradiation
exposure time.
Moreover, like in the Rothberg et al. work the rapid
decrease of the PL intensity, while the absorption
decrease slowly, shows that the PL intensity is
extinguished by the photochemically induced defects
(Rothberg, 1996). Perhaps FT-IR experiments in
function of irradiation exposure time corroborate
this supposition.
0 5 10 15 20 25 30 35 40 45
0
2
4
6
8
10
12
14
Experiment
Fit Exponential (2 Decay)
Integrate
d
PL
(
ar
b
. u.
)
Exposure time (hours)
600 650 700 750
0
1
2
3
4
5
6
7
8
9
PM MA/MEHPPV P L Spectra
0.00
0.25
0.50
0.75
1.00
2.00
6.00
11. 00
16. 00
21. 00
26. 00
31. 00
36. 00
41. 00
Intensity (arb. u.)
Wavelength (nm)
600650700750
0.0
0.2
0.4
0.6
0.8
1.0
1.2
0.00
1.00
2.00
16.00
26.00
PL Normalized Intensity
Wavelengt h (nm)
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
320

4 CONCLUSIONS
With the intention to develop long time irradiation
exposure blue-light dosimeters with greater light
emission efficiency, good mechanical properties and
safer for medical applications, in this paper was
studied the photodegradation of a photoluminescent
polymer blend composed by poly(methyl
methacrylate) (PMMA) as a host and Poly[2-
methoxy-5-(2-ethylhexyloxy)-1,4-
phenylenevinylene] (MEH-PPV) as a guest.
The results show that the PMMA/MEH-PPV
photoluminescent blend form a well structured film
with high homogeneity and high photoemission
intensity efficiency. Additionally, the PMMA/MEH-
PPV blend film photodegradation curve present a
biexponential time decay behavior with a long time
t
1
= 16.67 hours and a fast time t
2
=1.40 hours. These
results suggest that the PMMA/MEH-PPV blend
could be used as a long time irradiation exposure
blue-light dosimeter in the neonates treatment of
Hyperbilirubinaemia.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support
from CNPq grant 307266/2013-3, CAPES,
FAPEMIG and INFIS-UFU. We are grateful to Prof.
Noelio Oliveira Dantas that allowed the use of the
spectrofluorimeter, and Guilherme de Lima
Fernandes by his assistance with the AFM images.
REFERENCES
Yu, G., Heeger, A. J., 1996, in The Physics of
Semiconductors, Vol. 1 (Eds.: M. Schleffer, R.
Zimmerman), World Scientific, Singapore.
Hide F., Diaz Garcia, M. A., Schwartz, B. J., Heeger, A.J.
1997. New developments in the photonic applications
of conjugated polymers. Acc. Chem. Res., 30(10),
430–436.
Leclerc, M., 2001. Polyfluorenes: twenty years of progress.
J. Polym. Sci. Pol. Chem., 39(17), 2867–2873.
Silva, H. S., Nogueira, S. L., Manzoli, J. E., Neto, N. M.
B., Marletta, A., Serein-Spirau, F., Lère-Porte, J.P.,
Lois, S., Silva, R. A. 2011. Controlling Band gap
Energy and Multivibronic Modes of a Poly(2,5-
thiophene-1,4-dialkoxyphenylene) Derivative by
Gamma Photons. The Journal of Physical Chemistry.
A, 115, 8288-8294.
Ferreira, G. R., Nowacki, B., Magalhães, A., Azevedo, E.
R., de Sá, E. L., Akcelrud, L., Bianchi, R. F. 2014.
Controlling photo-oxidadtion processes of a
polyfluorene derivative: The effect of additives and
mechanism. Materials Chemistry and Physics, 146,
212-217.
Yan, M., Rothberg, L. J., Papadimitrakopoulos, F., Galvin,
M. E. and Miller T. M. 1994. Defect Quenching of
Conjugated Polymer Luminescence, Phys. Rev. Lett.,
73, 744.
Atreya, M., Li, S., Kang, E. T., Neoh, K. G., Ma, Z. H.,
Tan, K. L., Huang, W. 1999. Stability studies of
Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevi
nylene] [MEH-PPV]. Polymer Degradation and
Stability, 65, 287-296.
Yu, J., Hu, D. and Barbara, P. F. 2000. Unmasking
Electronic Energy transfer of Conjugated Polymers by
Suppression of O2 Quenching. Science, 289, 1327-
1330.
Jorgensen, M., Norrman, K., Krebs, F. C. 2008.
Stability/degradation of polymer solar cells. Solar
Energy Materials & Solar Cells, 92, 686-714.
Palacios-Lidon, E., Escasain, E., Lopez-Elvira, E., Baro,
A. M., Colchero, J. 2013. Photobleaching of MEH-
PPV thin films: correlation between optical properties
and nonoescale surface photovoltage. Solar Energy
Materials & Solar Cells, 117, 15-21.
Huser, T., Yan, M. 2001. Aggregation quenching in thin
films of MEH-PPV studied by near-field scanning
optical microscopy and spectroscopy. Synthetic
Metals, 116, 333-337.
Tozoni, J. R., Guimarães, F. E. G., Atvars, T. D. Z.,
Nowacki, B., Akcelrud, L., Bonagamba, T. J. 2009.
De-aggregation of polyfluorene derivative by blending
with a series of poly(alkyl methacrylate)s with varying
sidegroup sizes. Eur. Polym. J., 45(8), 2467–2477.
Spano, F. C., Silva, C. 2014. H- and J- Aggregate
Behavior in Polymeric Semiconductors. Annu. Rev.
phys. Chem., 65, 477-500.
Chou, H.L., Hsu, S.Y., Wei, P.K. 2005. Light emission in
phase separated conjugated and non-conjugated
polymer blends. Polymer, 46(13), 4967–4970.
Mirzov, O., Scheblykin, I.G. 2006. Photoluminescence
spectra of a conjugated polymer: from films and
solutions to single molecules. Phys. Chem. Chem.
Phys., 8(47), 5569–5576.
Lin, H., Hania, R. P., Bloem, R., Mirzov, O., Thomsson,
D., Scheblykin, I. G. 2010. Single chain versus single
aggregate spectroscopy of conjugated polymers.
Where is the border? Phys. Chem. Chem. Phys., 12,
11770–11777.
Soon, Y. W., Cho, H., Low, J., Bronstein, H., McCulloch,
I., Durrant, J. R. 2013. Correlation triplet yield, singlet
oxygen generation and photochemical stability in
polymer/fullerene blends films. Chem. Commun., 49,
1291-1293.
Lee, W., Park, H., Kwak, G. 2011. Solvent-assited,
accelerated photobleaching and fluorescence recovery
of conjugated polymer film. Chem. Commun., 47,
659-661.
Shoaee, S., Durrant, J. R. 2015. Oxygen diffusion
dynamics in organic semiconductor films. J. Mater.
Chem. C, 3, 10079-10084.
PMMA/MEH-PPV Photoluminescent Polymer Blend as a Long Time Exposure Blue-light Dosimeter
321

Rothberg, L.J., Yan, M., Papadimitrakopoulos, Galvin, M.
E., Kwock, E.W. and Miller, T.M. 1996, Photophysics
of phenylenevinylene polymers. Synthetic Metals, 80,
41-58.
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
322
