
Microfluidic Prototype of a Lab-on-Chip Device for Lung Cancer
Diagnostics
Dalila Vieira, Filipa Mata, Ana Moita and António Moreira
IN+, Instituto Superior Tecnico Universidade de Lisboa, Lisbon, Portugal
Keywords: Microfluidic Device, Electrowetting, Biofluid Dynamics, Wettability, Lung Cancer.
Abstract: Cell sorting for disease diagnostics is often achieved by fluorescence based identification of specific
markers. However, in lung cancer diagnostics, cytological analysis of pleural fluids is not always reliable
and immunofluorescence essays demand for specific sample preparation. Hence, this paper addresses the
development of a microfluidic device for lung cancer diagnostics which infers on the potential of a
diagnosis based on analysing the cell deformability (stiffness) that alters the rheological properties and
consequently the flow characteristics. Cell deformability will be induced by external actuation.
Electrowetting is used to transport the samples in an open configuration system using microdroplets. Effects
of the test chip configuration, sample physico-chemical properties and potential adsorption mechanisms are
discussed. Wettability plays here a vital role in the sample transport and in the diagnostic method to be
tested. Hence, an innovative approach is presented, the 3D Laser Scanning Fluorescence Confocal
Microscopy (3D-LSCFM) to provide a detailed reconstruction of the surface topology at the liquid-solid
interface region thus allowing contact angles measurement with high spatial resolution.
1 INTRODUCTION
Cell separation and sorting are critical in various
biomedical applications including diagnosis,
therapeutics and cell biology (Takahashi et al., 2004,
Gossett et al., 2010, Shields IV et al., 201,). Samples
of interest are often heterogeneous populations of
cells in a culture that comprises tissue. For instance,
the analysis of pleural fluid for lung cancer
diagnosis requires the previous separation of various
components, including blood cells (Gossett et al.,
2010). Although many standard techniques have
been developed for cell sorting, there are still several
challenges to overcome: they are often labour
intensive, require multiple additional tags or labels
to identify cells, have high costs, use large sized
equipment with low portability and require highly
skilled staff (Omori et al., 2015). Microfluidic
devices are pointed to be able to solve many of the
aforementioned problems and are actually
considered a fundamental pillar for the development
of point-of-care diagnostics (Yager et al., 2008).
Within this scope, this work aims at devising a
microfluidic chip for a lung cancer diagnosis. One
explores here the possibility to provide an earlier
diagnosis based on the deformability (stiffness) of
the cell, which alters the rheological properties and
consequently the flow characteristics. This new
approach follows the method suggested by (Gossett
et al., 2010), although these authors focused the
diagnostics on image analysis of the cells. Instead,
here, main emphasis is put on the cell deformation
inside microdroplets, as droplet spreading is
expected to be correlated to the rheological
modifications due to cells stiffness (Moita et al.,
2015). Microdroplets are also interesting regarding
sample handling in the microfluidic device. Sample
transport in continuous medium using microchannels
is the most popular approach in microfluidics, but
has problems associated with clogging, maintenance
and access to the samples. These difficulties can be
overcome with an open configuration system,
handling the samples in microdroplets, combining an
external actuation with custom made wetting
properties of the surfaces (Pollack et al., 2011, Moita
et al, 2016). However, external actuation (e.g.
electrostatic) may influence the internal droplet
flow, thus affecting droplet motion (Mugele, 2009).
Hence, a complete characterization of the flow is
required to tune the appropriate conditions for the
transport. Lab-on-a-chip open configuration systems
are still sparsely reported in the literature,
concerning the transport of biofluids. Several
Vieira D., Mata F., Moita A. and Moreira A.
Microfluidic Prototype of a Lab-on-Chip Device for Lung Cancer Diagnostics.
DOI: 10.5220/0006252700630068
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 63-68
ISBN: 978-989-758-216-5
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
63

authors report the effective electrowetting-induced
transport of physiological fluids, proteins and DNA
(Wheeler et al., 2005), but it is not clear which are
the most suitable electrochemical properties of the
fluids or the most important parameters governing
biofluids transport and manipulation. Adsorption of
the biocomponents on the dielectric substrate is also
a problem that is not completely understood yet and
its effects on the surface wettability are taken to a
secondary level. Yoon and Garrell (2003) showed
that protein solutions are often adsorbed by the
dielectric substrates. Recent work by Moita et al.
(2016) evidences that adsorption locally reduces the
contact angle, which aids droplet spreading but also
promotes energy dissipation at the contact line, thus
precluding droplet receding and making the droplet
transport more difficult, so this effect should not be
neglected. Finally, studies concerning EWOD
applications on microchips barely assess the
influence of design and configuration parameters. As
the droplet is transported on the chip surface, the
size and distance between electrodes must account
for the wetting properties and how they affect
droplet dynamics, which should be balanced by the
parameters affecting the electrical field. In this
context, for an initial stage of the development of the
microfluidic device, an optimization of the chip
configuration, taking into account the effect of the
liquid and surface properties is required.
Wettability plays a vital role in these flows, but
also here improved diagnostic techniques are
required to measure the micro-contact angles, which
can be significantly different form the apparent
angles typically obtained with tensiometers
(Sundberg et al., 2007, Vieira et al., 2016). In this
context, confocal fluorescence microscopy, which is
already used to infer on the potential adsorption
mechanisms is further explored here as a new
technique providing contact angle measurements
with high spatial resolution.
2 EXPERIMENTAL PROCEDURE
At this stage of the work, simple microfluidic chips
were devised to select the appropriate chip
configurations and materials and to infer on the
efficacy of the sample transport using
electrowetting. Biomimetic solutions of the pleural
fluid (as well as pleural fluid) are intended to be
used in the near future but for these preliminary tests
protein solutions and cell suspensions were used as
biofluid samples. GFP – Green Fluorescent Protein
(produced and purified in house) solution with
1.71x10
-3
mM concentration and GFP-expressing E.
coli suspensions with concentrations of 1x10
9
cells/ml and 2x10
9
cells/ml were the solutions
chosen, whose main physico-chemical properties,
namely density ρ, surface tension σ
lv
and dynamic
viscosity μ are summarized in Table 1. Regarding
their rheology, all the fluids used here are
Newtonian.
Table 1: Physico-chemical properties of the biofluids.
Solution
Density ρ
[kg/m
3
]
Surface
tension σ
lv
[mN/m]
Dynamic
viscosity μ
[Ns/m
2
]
GFP (1.71x10
-3
mM)
998
72.2±0.7
1x10
-3
GFP-expressing
E. coli (1x10
9
cells/ml)
998
73.8±0.04
1x10
-3
GFP-expressing
E. coli (2x10
9
cells/ml)
998
73.8±0.04
1x10
-3
For the measurements with the 3D Laser
Scanning Fluorescence Confocal Microscopy – 3D
LSCFM a fluorescent dye - Rhodamine B (Sigma
Aldrich) is used, which was chosen taken into
account its excitation and emission wavelengths, to
be compatible with the wavelengths available in the
Laser Scanning Confocal Microscope (Leica SP8),
but also due to particular characteristics of the
experimental conditions, in the present study. For
the concentrations used here
(0.0007936mg/ml<Concentration<0.496mg/ml) the
physico-chemical properties of the water-dye
solutions are very close to those of water. Detailed
description of the measurement procedures is
provided in Vieira et al. (2016).
The test chips, manufactured at INESC-MN are
printed by lithography and the patterned transferred
by wet etch. Finally, a thin film of a dielectric
material is deposited on the chip assembly. The
chips mainly comprise numerous interdigited
electrodes displaced with a fixed distance of 60μm
between them. The variable in the chips
configuration is the width of the electrodes, which
varies between 80μm and 1400μm. The length of the
electrodes is 24mm. The usable chip area is
32x22mm
2
. The applied voltage is varied from 0 to
250V, also to infer on its influence on the droplet
motion. The frequency was varied between 50Hz
and 450Hz. To infer on the possible adsorption of
the biocomponents on the dielectric substrates over
which the droplets of the biofluids are transported,
simple tests are performed in which droplets of the
biofluids are deposited on the surfaces. Afterwards,
BIODEVICES 2017 - 10th International Conference on Biomedical Electronics and Devices
64

a sequence of tests with and without electrostatic
actuation is performed and the “footprints” of the
droplets are observed on the Laser Scanning
Confocal Microscope (Leica SP8). The obtained
images are then post-processed to determine the
mean grey intensity (sum of intensities divided by
the number of pixels in the region of interest of the
droplet footprint) and the Area Integrated Intensity
(sum of intensities of pixels in the region of interest
of the droplet footprint normalized by unit of area
(μm
2
). Since the droplet spreads after actuation, the
integrated density is weighted with the area. To
reduce the noise, the average grey intensity levels of
the background image were also subtracted. The
final result is the herein so-called Total Corrected
Droplet Fluorescence – TCDF, as proposed by
Moita et al. (2016). Higher values of TCDF can be
associated to a larger quantity of the protein or cells
adsorbed by the substrate. The wetting properties of
the dielectric substrates play a vital role. Different
chemical and topographical characteristics are tested
to infer on the most favourable to handle the
biosamples in the various sections of the
microfluidic device. The topography is measured
using a Dektak 3 profile meter (Veeco) with a
vertical resolution of 20nm.
The wettability of the dielectric substrates is
quantified with an optical tensiometer (THETA from
Attention), by the static contact angle θ
e
and by
hysteresis. Given the relatively low resolution of
these measurements and considering the typical
scale of the processes governing the transport of the
samples within the droplets (micro-to-nano scale) an
alternative method is explored to provide more
accurate measurements. Detailed description of this
technique can be found in Vieira et al. (2016).
Finally, transport and deformation of the biofluid
droplets are characterized based on high-speed
visualization at 2200 fps using a Phantom v4.2 from
Vision Research Inc., with 512x512 pixels@2100fps
resolution. Post-processing home-made routines are
then used to measure deformability, spreading
diameters, velocity of droplets transport and
displacement velocity of the contact line (droplet-
dielectric substrate).
3 DEVICE CONFIGURATION
The microfluidic device under development has
three main working sections, namely, the transport
section, the diagnostic section and the
sorting/selection section. In the transport section,
which enables the transport of the biofluid droplet to
the diagnostic section, droplet motion is governed by
electrostatic actuation aided by custom made wetting
properties of the dielectric material. In this section,
hydrophobic/superhydrophobic regions are preferred
to minimize the adhesive forces, which are
proportional to hysteresis and consequently
minimize the energy dissipated at the contact line
between the droplet and the surface. Similar wetting
properties are required in the sorting/selection
section. On the other hand, in the diagnostic section
it worth promoting adhesion to constrain the sample
in the sensor area. The diagnostic methodology to
explore considers the correlation of different ratios
of cell stiffness with the degrees of the pathology,
being expected to be able to detect cell malignancy
at very early stages (Gosset et al, 2010). The
deformation of the cells inside the microdroplets is
induced. Different approaches will be explored to
impose the deformation but at this stage of the work,
electrostatic actuation is being tested. A numerical
model is being developed at two scales. At the
mesoscale, the deformability of the cell is simulated.
At this scale surface wettability effects, such as
adhesion and repulsion, are related to molecular
bonding and Van de Walls forces. The main outputs
of this model are the forces that must be exerted to
impose the various degrees of deformability of the
cell to validate the diagnostic method. On the other
hand, despite being a macroscopic flow, the
spreading of a microdroplet is known to be very
sensitive to small rheological modifications of the
fluid and can be correlated with the parameters
governing the constitutive models (Moita et al.,
2015). Hence droplet spreading is expected to be
correlated to the various levels of cell stiffness.
These levels of cell stiffness can be grouped in
classes of internal cell viscosity/water viscosity and
correlated to different degrees of the pathology. At
this scale, surface wettability can simply be
modelled based on interfacial tensions, hysteresis
and contact angle values, but still requires very
accurate contact angle measurements combined with
a detailed characterization of the droplet internal
flow, particularly near the surface.
Following the discussion in the previous
paragraphs, the selection of the materials to use as
the dielectric film, plays here a determinant role, as
different sections of the microfluidic chip demand
for opposite wetting regimes. The first approach
considered here was tailoring surface topography to
alter the wettability. Hence, photolithography was
used to define micro-patterns of regular
cavities/pillars. For an accurate measurement of the
contact angles and clearer description of the contact
Microfluidic Prototype of a Lab-on-Chip Device for Lung Cancer Diagnostics
65
line, the contact angles were with a new technique,
the 3D LSCFM, described in the next paragraphs.
3.1 Contact Angle Measurements with
3D LSCFM
To validate and explore the 3D LSCFM technique,
preliminary results were obtained by measuring the
equilibrium contact angles on smooth glass slides.
The equilibrium angles are measured for millimetric
(with diameters of 3mm) and micrometric (with
diameters ranging between tens of microns up to
1mm) droplets, to infer on their dependence on
droplet size. The obtained measures are compared
with those obtained with the optical tensiometer
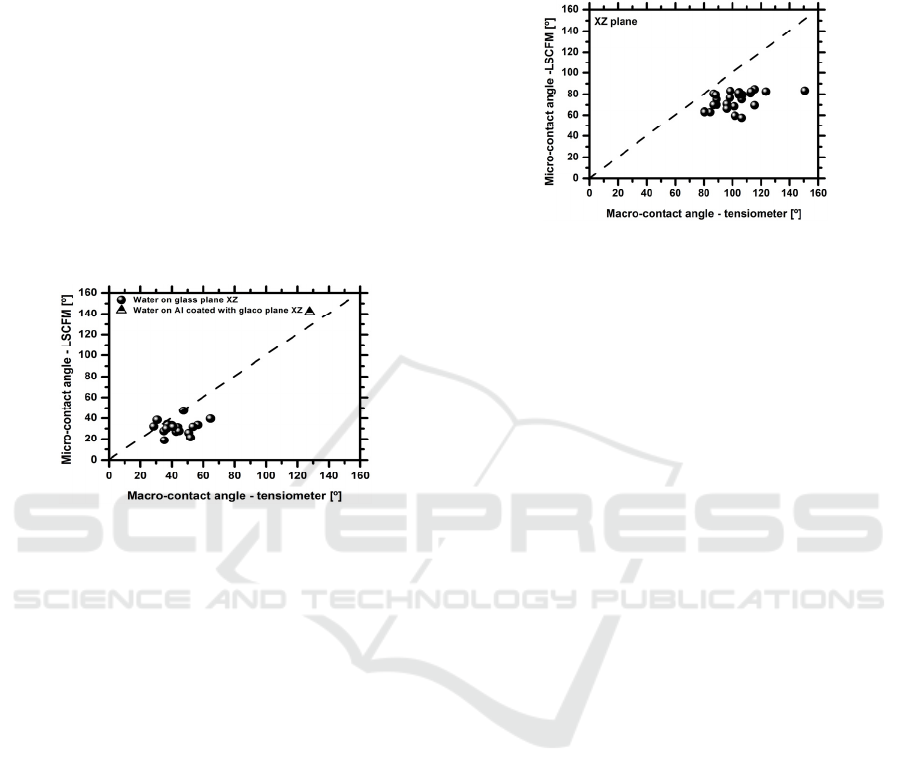
(Figure 1).
Figure 1: Comparison between the equilibrium angles
measured with tensiometer and with the LSCFM
technique, measured for a smooth glass and coated Al
surfaces.
Overall the results show that both techniques
provide similar measurements, although the values
obtained from the LSCFM tend to be lower, when
compared to those given by the optical tensiometer.
This is due to the scale and resolution that are being
considered in the LSCFM. Hence, the error
associated to the worse resolution used in the
LSCFM is of 1.87µm (with negligible propagation
to the angle measurement) against 156µm obtained
with the optical tensiometer. So a more detailed
view of the contact line region can be obtained with
the 3D LSFCM, which consequently provides a
more accurate shape of the region defining the
contact angle. These observations are qualitatively in
agreement with those reported by Salim et al.
(2008).
Once the measurement technique is validated, it
can be applied to more complex surfaces, namely
those with patterned micro-structures. For these
surfaces a large difference is observed between the
measures obtained with the optical tensiometer and
those taken with the 3D LSCFM technique, as
depicted in Figure 2. Hence, there are apparent
angles of 120º, as measured by the tensiometer, thus
evidencing a hydrophobic behavior, which are in
fact up to 40º lower, when measured with the 3D
LSCFM technique.
Figure 2: Comparison between the equilibrium angles
measured with the optical tensiometer and with the
LSCFM technique measured on micro-textured silicon
wafer surfaces.
The lower angles measured with the 3D LSCFM
are in agreement with the observations of the contact
line region. So, the apparent hydrophobic regime of
some of the surfaces characterized with the
tensiometer is not accurate and often not stable, as
the liquid droplet sags in between the surface
patterns. Furthermore, the 3D reconstructions of the
droplet on both XZ and YZ planes show an evident
distortion of the contact line. These observations of
the contact line may also support our previous
results (e.g. Moita et al 2016) which showed that
surface topography in these non-stable hydrophobic
surfaces promotes energy dissipation at the contact
line, thus precluding droplet motion. In line with
these results, the safest way to alter wettability, for
the current stage of development of the work is
towards the chemical modification and/or selection
of the appropriate dielectric materials, as discussed
in the following sub-section.
3.2 Selection of the Materials as a
Function of the Influencing
Parameters
The selection of the dielectric materials to use
should be based on the contact angle measurements
but also on the hysteresis, which should be the
smallest possible, since the resistance force opposing
to droplet motion is proportional to the surface
tension σ
lv
and to the hysteresis. In this context
Table 2 depicts the equilibrium angles, obtained for
each biofluid tested, on various dielectric coatings
which are commonly used in electrowetting chips.
Water is used as reference. The Table shows that
only the SU8 resist and Si
3
N
4
surfaces are
BIODEVICES 2017 - 10th International Conference on Biomedical Electronics and Devices
66
hydrophilic, being the others hydrophobic. The
highest contact angle of 121º is obtained for the
PDMS substrate. Despite having high contact
angles, which is desired for the transport of the
samples, PDMS and Teflon substrates depict also
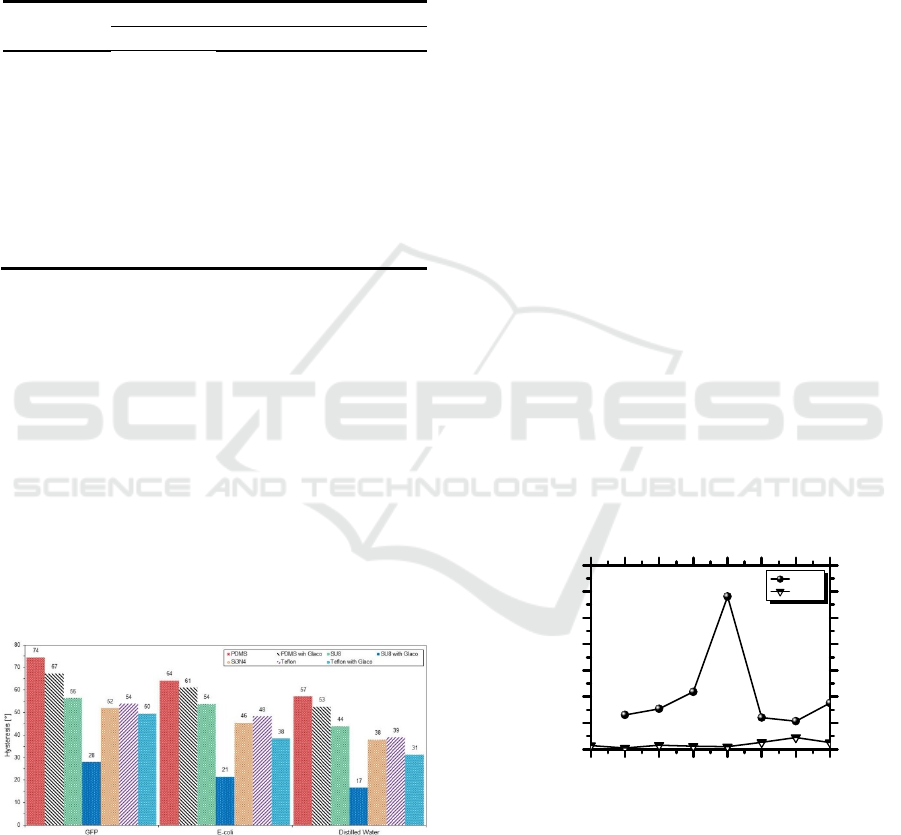
high hysteresis (Figure 3).
Table 2: Equilibrium contact angles, obtained for each pair
fluid-dielectric substrate considered in the present work.
Dielectric
coating
Contact angle [°]
Water E-coli GFP
Teflon 112±5 103±6 121±6
Teflon with
Glaco
145±1 141±9 153±3
PDMS 121±1 112±1 119.5±0.4
PDMS with
Glaco
153±3 153±2 155±3
SU8 resist 67.1±0.7 65±2 71.8±0.2
SU8 with
Glaco
160±7 162±1 153±4
Si
3
N
4
64.1±0.7 59±4 65±2
Glaco® is a commercial coating which is mainly
a perfluoroalkyltrichlorosilane combined with
perfluoropolyether carboxylic acid and a fluorinated
solvent (Kato et al., 2008). Its application allows
obtaining superhydrophobic surfaces with high
contact angles (>150º) and low hysteresis (<10º)
being therefore a good option to consider in the
transport section of the microfluidic device.
Concerning adsorption, Moita et al. (2016) report
that the GFP protein was adsorbed by Teflon
substrates, leading to a local increase of surface
wettability and further contributing to preclude the
receding motion, as this wettability increase is
irreversible, taking the contact angles to values near
saturation.
Figure 3: Contact angle hysteresis evaluated for GFP
solution (1.71x10
-3
mM), GFP-expressing E-coli
suspension (1x10
9
cells/mL) and distilled water on the
tested dielectric substrates.
In this context, this work infers on the possible
adsorption of the GFP and E-coli cells by the
substrates, evaluated by the TCDF value. The results
evidenced a minimum value of TCDF =
8.88x10
7
and TCDF = 1.65x10
7
obtained for the
adsorption of GFP (1.71x10
-3
mM) and E-Coli
suspension (1x10
9
cells/mL) on PDMS, respectively.
The alternative material with lower adsorption of the
biocomponents tested here was SU8, but the TCDF
values obtained were about one order of magnitude
higher than those evaluated for PDMS. Hence,
PDMS is a good choice. In addition, the application
of Glaco® coating is observed to further reduce the
adsorption of both proteins and cells, decreasing the
TCDF values in about one order of magnitude. Once
the material is selected, its effect must be further
evaluated in the dynamic response of the droplet and
its deformability, which also depends on the
properties of the biofluids. For illustrative purposes
and due to paper length constrains, Figure 4 depicts
the effect of the biofluid properties on droplet
motion on electrowetting (in the transport section),
evaluated based on its maximum spreading diameter,
made non-dimensional with the initial droplet
diameter as it is collected on the surface, d
max
/d
0V
.
The Figure evidences the better response of the
droplets of protein solutions, although the surface
tension and density only vary slightly between the
different solutions (Table 1). Since the cellular
compounds are bigger and heavier than the proteins,
they should be more difficult to carry. In fact, the
cells have a greater propensity to adhere to the
surface and tend to agglomerate, increasing the local
density and ending up creating resistance to motion.
All these aspects must be considered in the
following new configurations of the microfluidic
chip, once the materials are selected.
Figure 4: Maximum spreading dimensionless diameter of
GFP (1.71x10
-3
mM) and GFP-expressing E-coli
suspension (1x10
9
cells/mL), for droplets moving between
coplanar electrodes (transport section of the device).
4 SUMMARY
The present paper presents the preliminary stages of
development of a microfluidic device for lung
50 100 150 200 250 300 350 400
1.00
1.05
1.10
1.15
1.20
1.25
1.30
1.35
d
max
/d
0V
[-]
Fequency [Hz]
GFP
E. Coli
Microfluidic Prototype of a Lab-on-Chip Device for Lung Cancer Diagnostics
67

cancer diagnostics which infers on the potential of a
diagnosis based on analysing the cell deformability
(stiffness). The cell stiffness is expected to alter the
rheological properties and consequently the flow
characteristics in a detectable way, which is
correlated with cell malignancy. The main concepts
behind this new diagnostic method are explained
together with the global description of the
microfluidic device. Given the important role of the
wettability, a new methodology is explored here to
obtain contact angle measurements with high spatial
resolution. At this preliminary stage of the work, the
importance of the wettability is discussed in the
selection of the materials. The dynamic response of
different biofluids is also briefly discussed.
ACKNOWLEDGEMENTS
The authors are grateful to Fundação para a Ciência
e a Tecnologia (FCT) for partially financing this
research through the project UID/EEA/50009/2013,
which also supports Dalila Vieira with a fellowship.
The work was also partially financed by FCT
through the project RECI/EMS-SIS/0147/2012,
which also supported Filipa Mata with a fellowship.
A.S. Moita also acknowledges the contribution of
FCT for financing her contract through the IF 2015
recruitment program. Finally, the authors
acknowledge the contribution of Joana Pereira in the
data acquisition and post-processing of the 3D-
LSCFM data.
REFERENCES
Gossett, D., Weaver, W., Mach, A., Hur, S., Tse, H., Lee,
W., Amini H., Carlo, D., 2010. Label-free cell
separation and sorting in microfluidic systems,
Analytical and Bioanalytical Chemistry, 397:3249-
3267.
Kato M, Tanaka A, Sasagawa M, Adachi H, 2008.
Durable automotive windshield coating and the use
thereof. US Patent, 8043421 B2.
Moita, A. S., Laurência, C., Ramos, J.A., Prazeres, D. M.
F., Moreira, A. L. N., 2016. Dynamics of droplets of
biological fluids on smooth superhydrophobic surfaces
under electrostatic actuation, J. Bionic Eng., 13:220-
234.
Mugele, F., 2009. Fundamental challenges in
electrowetting: from equilibrium shapes, to contact
angle saturation and drop dynamics, Soft Materials,
5:3377-3384.
Omori, T., Imai, Y., Kikuchi, K., Ishikawa, T.,
Yamaguchi, T., 2015. Hemodynamics in the
Microcirculation and Microfluidics, Annals of
Biomedical Engineering, 43:238-257.
Pollack, M. G., Pamula, V.K., Srinivasan, V., Eckhardt,
A.E., 2011. Applications of electrowetting-based
digital microfluidics in clinical diagnostics, Expert
Ver. Molecular Diagnostics, 11(4):397-407.
Salim, A., Sausse, J., Pironon, J., Fourar, M., Le Carlier
De Veslud, C., 2008. 3D confocal laser microscopy to
quantify contact angles in natural oil-water mixtures,
Oil & Gas Sci Tech. Rev. IFP, 63(5):645-655.
Shields IV, C. W., Reyes, C. D., López, G. P., 2015.
Microfluidic Cell Sorting: A Review of the Advances
in the Separation of Cells from Debulking to Rare Cell
Isolation, Lab on a Chip, 16:1230–1249.
Sundberg, M., Mansson, A., Tagerud, S., 2007. Contact
angle measurements by confocal microscopy for non-
destructive microscale surface characterization, J.
Coll. Int. Sci., 312:454-460.
Takahashi, K., Hattori, A., Suzuki, I., Ichiki, T., 2004.
Non-destructive on-chip cell sorting system with real-
time microscopic image processing, Journal of
Nanobiotechnology, 2:1-8.
Vieira, D., Moita, A. S., Moreira, A. L. N., 2016. Non-
intrusive wettability characterization on complex
surfaces using 3D Laser Scanning Confocal
Fluorescence Microscopy, 18th International
Symposium on Applications of Laser and Imaging
Techniques to Fluid Mechanics, Lisbon.
Wheeler A R, Moon H, Kim C J, Loo J A, Garrell R L.,
2004. Electrowetting-based microfluidics for analysis
of peptides and proteins by matrix-assisted laser
desorption/ionization mass spectrometry. Analytical
Chemistry, 76, 4833–4838.
Yager, P., Domingo, G., Gerdes, J., 2008. Point-of-care
diagnostics for global health, Annu. Rev. Biomed.
Eng., 10:231-240.
Yoon J Y, Garrell R L., 2003. Preventing biomolecular
adsorption in electrowetting-based biofluidic chips.
Analytical Chemistry, 75, 5097–5102.
BIODEVICES 2017 - 10th International Conference on Biomedical Electronics and Devices
68
