
Sharing of Big Data in Healthcare: Public Opinion, Trust, and Privacy
Considerations for Health Informatics Researchers
Laura Moss
1,2
, Martin Shaw
2
, Ian Piper
2
, Christopher Hawthorne
3
and John Kinsella
1
1
Department of Anaesthesia, Pain & Critical Care, School of Medicine, University of Glasgow, Glasgow, U.K.
2
Department of Clinical Physics, NHS Greater Glasgow & Clyde, Glasgow, U.K.
3
Department of Neuroanaesthesia, NHS Greater Glasgow & Clyde, Glasgow, U.K.
Keywords:
Big Data, Privacy, Critical Care, Data Analysis, Trust, Data Security.
Abstract:
Advances in technology has transformed clinical medicine; electronic patient records routinely store clinical
notes, internet-enabled mobile apps support self-management of chronic diseases, point-of-care testing enables
laboratory tests to be performed outside of hospital environments, patient treatment can be delivered over wide
geographic areas and wireless sensor networks are able to collect and send physiological data. Increasingly,
this technology leads to the development of large databases of sensitive electronic patient information. There
is public interest into the secondary use of this data; many concerns are voiced about the involvement of private
companies and the security and privacy of this data, but at the same time, these databases present a valuable
source of clinical information which can drive health informatics and clinical research, leading to improved
patient treatment. In this position paper, we argue that for health informatics projects to be successful, public
concerns over the secondary use of patient data need to be addressed in the design and implementation of the
technology and conduct of the research project.
1 INTRODUCTION
Healthcare is rapidly changing and advanced technol-
ogy is enabling the collection of vast amounts of pa-
tient data. Consequently healthcare is experiencing
a Big Data phenomenon. Whilst health informatics
research is focused on the development of novel ap-
proaches to enable the intelligent analysis of this data,
the use of electronic patient data to advance these ap-
proaches and the implementation of these technolo-
gies within real world healthcare environments raises
many ethical questions and divides public opinion.
In this paper we explore some of the issues regard-
ing the use of electronic patient data for secondary
purposes, specifically trust, security and patient con-
fidentiality. The paper is organised as follows: section
2 describes the prominence of Big Data within health-
care and challenges faced; section 3 discusses issues
raised in the sharing of data, in particular the involve-
ment of private companies and views of the general
public; section 4 illustrates real-world issues faced
whilst implementing a big data analysis platform into
a healthcare environment; section 5 discusses consid-
erations and opportunities for health informatics re-
searchers; finally, section 6 concludes the discussion.
2 BACKGROUND
As technology advances, data is increasingly col-
lected through a variety of mechanisms. It is esti-
mated that 2.5 quintillion bytes of data is currently
generated each day (IBM a, 2016). Big Data is a term
which is now commonly used to describe such large
and complex datasets. Advanced analytics are often
applied to Big Data to extract meaning, insights and
discovery of new knowledge. However, using these
large datasets presents challenges due to the data’s
volume (many sources e.g. sensors, social network-
ing), variety (many formats, e.g. videos, text) and ve-
locity (speed at which data is produced and require-
ments for near-real time processing). Other attributes
can include veracity (noisy, messy data), variability
(meaning of the data can be constantly shifting) and
fine-grained (Kitchen and McArdle, 2016).
Within healthcare, the increasing use of technol-
ogy is creating large volumes of clinical data; in 2011,
the global size of healthcare data was estimated to be
161 billion gigabytes (IBM b, 2016). This influx of
data is from a variety of technical advances: e.g. en-
hanced clinical imaging, electronic medical records,
and physiological sensors. Additionally, the growth
Moss L., Shaw M., Piper I., Hawthorne C. and Kinsella J.
Sharing of Big Data in Healthcare: Public Opinion, Trust, and Privacy Considerations for Health Informatics Researchers.
DOI: 10.5220/0006251504630468
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 463-468
ISBN: 978-989-758-213-4
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
463

of wearable sensor devices (e.g. smart watches) is
leading to patients themselves being able to generate
large amounts of healthcare data.
The potential clinical benefit of using this data
is well established. Analysis of healthcare data can
drive improvement in many areas, including: clini-
cal and organisation processes, optimisation of treat-
ments, and reduction of healthcare costs through in-
tervention at an earlier point and more proactive and
targeted care. Patient data can be used to predict clin-
ical risk, targeting resources where they are needed
most and identifying problems that would benefit
from early intervention. Technologies that aid clin-
ical decision-making and help clinicians to manage
the exponential growth in medical knowledge offer
substantial opportunities to reduce variation, improve
the quality of care, and possibly lower costs (Jaspers
et al., 2011), (Fillmore et al., 2013).
Whilst a thriving health informatics community is
focused on the development of novel approaches and
tools to enable the intelligent and sophisticated anal-
ysis and use of healthcare data, the transfer of these
technologies and ideas to real world clinical environ-
ments faces many challenges. Healthcare providers
often do not have access themselves to data sci-
ence expertise, required technology infrastructure and
funding to do this themselves. In many cases, to en-
able the potential for big data analysis to improve
healthcare, partnerships have to be formed between
healthcare providers, research organisations and pri-
vate companies. High profile examples include Deep-
Mind and IBM Watson technology. IBM’s Watson is
being used within oncology in US and Canadian hos-
pitals to assess tumours (IBM c, 2016) and DeepMind
is using patient data from UK hospitals to develop
diagnostic tools in areas such as acute kidney injury
(DeepMind a, 2016) and ophthalmology (DeepMind
b, 2016).
3 TRUST, SECURITY AND
PRIVACY
Trust and confidentiality between a clinical provider
and patient is not new: it is central to the practice
of healthcare and has been focused on since Hip-
pocrates. Whilst the concept of patient confidential-
ity has endured as an ideal throughout history, the
precise nature of it has changed with the sociohis-
toric context (for a detailed review see (A.Ferguson,
2012)). In the digital age, patient confidentiality is
often framed within the context of electronic patient
records and the potential involvement of third parties.
Whilst the involvement of private organisations and
other research organisations can resolve many prac-
tical issues for healthcare providers, it often involves
the transfer of sensitive patient data to these organ-
isations for research development and this can raise
many questions. For example:
• Should electronic patient data be used for sec-
ondary research purposes?
• Who does the electronic patient data belong to?
• Who should be able to use the electronic patient
data? Public sector healthcare organisations (e.g.
NHS) and/or commercial companies?
• Who is collecting the data and where is it stored?
• What safeguards should be put in place to protect
patient confidentiality?
• How do patients and relatives feel about the col-
lection and use of this data?
Most countries have some form of regulation or
processes which have been legally put in place to re-
solve some of the above issues. Additionally, insti-
tutions often have internal procedures and practices
in place to protect patient data. However, public
perception of the adequacy of these frameworks can
be divided and concerns raised about whether pri-
vate companies, in particular, can be trusted with pa-
tient data. For example, the public sector can be per-
ceived as more trustworthy than profit-making organ-
isations when using patient data (Focus Groups et al.,
2013). Recent publicity in cases such as DeepMind’s
arrangement with the Royal Free Hospital, London,
has been controversial and highlighted the require-
ment for more robust safeguards to be put in place to
ensure patient data is adequately secured (New Sci-
entist, 2016). Additionally, there are constant reports
in the media about data leaks and unsuccessful, large-
scale, health I.T projects (Presser et al., 2015).
It also raises issues about whether patient con-
sent and public awareness of healthcare data shar-
ing is adequate. A number of studies have identified
that there is low awareness by the general public of
electronic patient record systems and how and why
healthcare data might be used (BMA, 2016) (Rior-
dan et al., 2015). Riordan et al (Riordan et al., 2015)
found that most people would prefer to opt-in before
their identifiable records were used and half of partic-
ipants would share their de-identified records under
implicit consent. A recent consultation with the gen-
eral public in the UK on this issue identified that they
had little confidence in the safeguards put in place to
protect data. Additionally, it was felt that there was
a lack of accountability within the system, and ma-
licious use of data by private companies (e.g. phar-
maceutical and insurance) was a concern for many
HEALTHINF 2017 - 10th International Conference on Health Informatics
464

people (BMA, 2016). Lessons learnt from the fail-
ure of large scale patient data sharing projects show
the requirements for clear communication to the pub-
lic, easy to understand consent rules and strong over-
sight and communication regarding distribution and
use of patient data (Presser et al., 2015). For a more
detailed systematic review of the literature regarding
public responses to sharing of health data see (Aitken
et al., 2016b).
To investigate the opinions of the general pub-
lic further, in a small study we asked the audience
at a science festival their thoughts on the topic of
secondary use of patient data (Kinsella et al., 2017).
Questions covered included: whether the participants
were aware of the potential of using their medical
data for secondary research purposes; whether pa-
tient data should be used for research purposes and
how likely would they be themselves to share their
own personal data for research; if they trust clinicians
with their data; and their opinions on the role of pri-
vate/commercial companies in supporting and/or car-
rying out research on their own medical data. 39 out
of 41 adults responded to the survey (from which we
have full results for 37 adults). Table 1 shows re-
sults from the survey. The vast majority of respon-
dents felt that their medical data should be used for
research purposes and would be happy to share their
data. This is in keeping with a number of other studies
(as summarised in (Aitken et al., 2016b). Addition-
ally, most respondents trusted clinicians, but when it
came to private companies, the response was mixed.
This difference in perceived trustworthiness between
clinicians and private companies has been found pre-
viously (e.g.(Aitken et al., 2016a),(BMA, 2016)).
We also looked for any age divide in the partici-
pant’s responses. We asked which age category par-
ticipants fell into (21 and under, 22-34, 35-44, 45-
54, 55-64, 65 and over) and three questions regarding
the involvement of private companies: 1) Would you
trust a private company to do research with your med-
ical data? 2) Would you trust a private company to
do research with anonymous medical data? 3) Would
you be comfortable with a private company provid-
ing the support to medical researchers to enable them
to do medical research? Tables 2 and 3 display the
results of this analysis. In both age groups, more peo-
ple had positive responses (agreed or strongly agreed)
than negative ones (disagree or strongly disagree). Al-
though younger people are often thought to be more
confident with technology and data sharing, no siz-
able difference between the two age groups was found
in this study. A larger sample size would be required
to show statistical significance.
Public opinion and perception of the use of health-
care data can be divided and it is clear from these stud-
ies and the opinions of other researchers (e.g. (van
Staa et al., 2016)), that for health informatics projects
involving transfer and analysis of patient data by third
parties to be a success, the trust of the general public
needs to be earned and respected by all involved.
4 CHART-ADAPT CASE STUDY
To illustrate some of the issues which may need to
be considered in collaborative health informatics re-
search projects, in this section we discuss some of the
actions which the CHART-ADAPT project instigated
to try and overcome data sharing concerns (CHART-
ADAPT, 2016).
The CHART-ADAPT platform allows the fast
analysis of high and low frequency data collected
from a critical care unit; enabling the creation and
assessment of novel, closed loop, diagnostic or ther-
apeutic models and algorithms. Routinely recorded
patient data is automatically transferred from the elec-
tronic patient record system in the critical care unit to
a high performance computing platform implement-
ing a Spark (Apache a, 2016), Scala (Scala, 2016) and
Hadoop (Apache b, 2016) technology stack. Complex
physiological algorithms are then applied to the data
to derive clinically useful variables which are returned
back into the clinical environment and integrated with
the existing electronic patient record system.
A lack of the required technical infrastructure
within the hospital to process the patient data within
clinically meaningful timescales meant it was es-
sential for the healthcare provider and academic re-
searchers to form a collaborative team which included
commercial partners to provide the required high per-
formance computing infrastructure.
Due to the nature of the collaboration and the re-
quirement to transfer patient data, the project team
was aware of the need to maintain patient confi-
dentiality, the governance of patient data, and the
need to gain the confidence of patients and unit
staff. Several concerns were identified: 1) failure of
the de-identification software and subsequent trans-
fer of identifiable patient data outside the health-
care provider’s network, 2) secure handling of the
anonymised patient data by the commercial partner,
3) correct re-identification of patient data when it re-
entered the healthcare provider’s network, and 4) pub-
lic perception of a commercial partner supporting the
patient data analysis. These concerns were considered
from the start of the project and all the collaborating
organisations worked together to integrate the follow-
ing activities into the project plan:
Sharing of Big Data in Healthcare: Public Opinion, Trust, and Privacy Considerations for Health Informatics Researchers
465
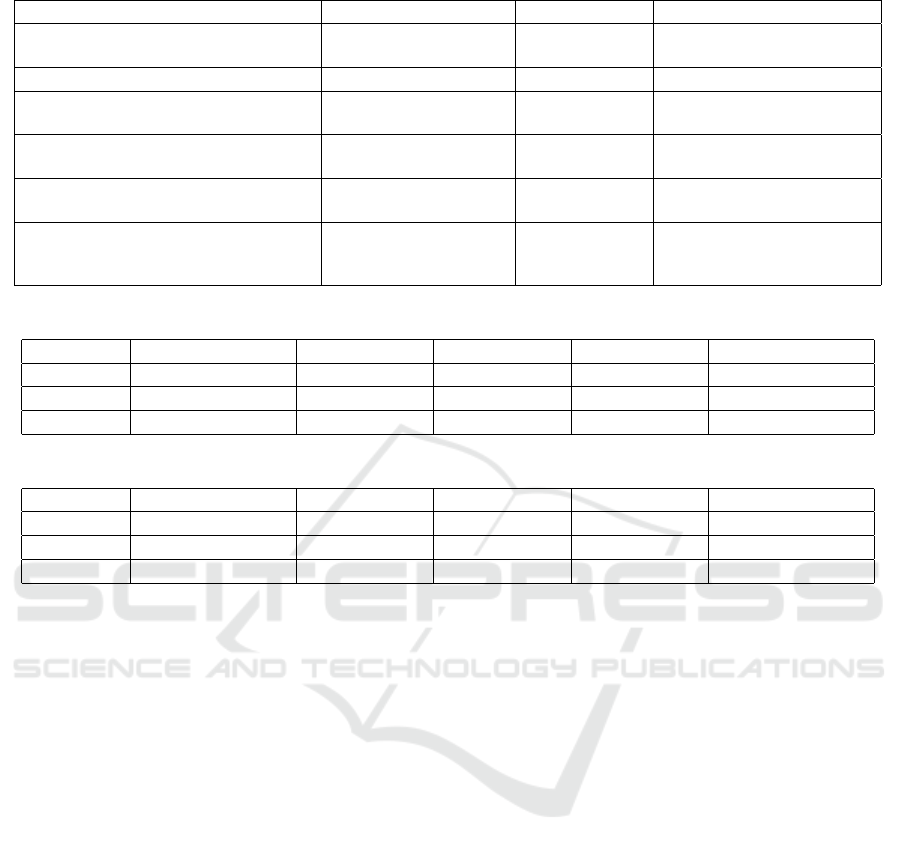
Table 1: General Public Opinions on Secondary Healthcare Use.
Question Yes No
Are you aware that medical data could
be used for research?
30 (81.2%) 7 (18.9%)
Question Strongly agree/agree Undecided Disagree/Strongly disagree
Medical data should be used for re-
search
33 (89.2%) 4 (10.8%) 0
Would you be happy to share your
healthcare data?
31 (83.8%) 4 (10.8%) 2 (5.4%)
Do you trust clinicians with your
healthcare data?
27 (73%) 7 (18.9%) 3 (8.1%)
Do you trust private companies to use
your medical data for research pur-
poses?
8 (21.6%) 20 (54%) 9 (24.3%)
Table 2: Responses < 35 years old, Total = 21 respondents.
Question Strongly Agree Agree Undecided Disagree Strongly Disagree
1 1 (4.8%) 7 (33.3%) 10 (47.6%) 2 (9.5%) 1 (4.8%)
2 4 (19%) 8 (38.1%) 7 (33.3%) 1 (4.8%) 1 (4.8%)
3 5 (23.8%) 10 (47.6%) 5 (23.8%) 0 (0%) 1 (4.8%)
Table 3: Responses >= 35 years old, Total = 16 respondents
Question Strongly Agree Agree Undecided Disagree Strongly Disagree
1 1 (6.25%) 3 (18.8%) 8 (50%) 2 (12.5%) 2 (12.5%)
2 2 (12.5%) 7 (43.8%) 5 (31.3%) 2 (12.5%) 0 (0%)
3 4 (25%) 5 (31.3%) 6 (37.5%) 1 (6.25%) 0 (0%)
• Regulatory approval (beyond minimum require-
ments) was acquired for the transfer of pa-
tient data (e.g. NHS Research Ethics, Caldicott
Guardian approval)
• The project developed software to automatically
anonymise the patient data before it left the
healthcare environment. A rigorous testing plan
was followed and repeated at regular intervals to
ensure confidentiality was maintained.
• The commercial partner responsible for techni-
cal support of the data analysis (Aridhia) devel-
oped an Information Governance Strategy for the
project which made explicit the data handling and
security procedures put in place. Close communi-
cation was also maintained between the personnel
responsible for Information Governance in both
organisations (healthcare and commercial).
• Public engagement initiatives were implemented.
For example, posters and leaflets were made avail-
able in the unit, staff were briefed and updated on
project progress, and a public event was hosted to
discuss patient data sharing within critical care.
• Attendance at relevant academic and healthcare
events was scheduled into the project. This gave
the team the opportunity to discuss the platform
and gather feedback which was fed into the devel-
opment of the project.
Although some activities were time-consuming
and beyond the usual scope of a research project, it
was beneficial not only for development of the plat-
form, but also to make sure, to aid future acceptance
of the technology, that we took the clinical staff and
general public with the project, rather than exclude
them and present the technology as a fait accompli.
5 OPPORTUNITIES FOR
HEALTH INFORMATICS
RESEARCH
Whilst public opinion on trust, security and privacy
of patient data needs be carefully considered in re-
search projects, there is also an opportunity for the
health informatics community to develop tools and
technologies to address these concerns. Below are
some suggestions (although this is not exclusive) and
comments on how the health informatics community
may be able to contribute:
• Communication of Health Informatics Projects
- There is a need to develop clear, concise, up-to-
HEALTHINF 2017 - 10th International Conference on Health Informatics
466

date summaries of health informatics projects to
aid transparency, in particular regarding the use
of patient data. Researchers should consider how
they will engage the public when designing and
implementing the health informatics project.
• Dynamic Consent - Current mechanisms of in-
formed consent for patient data sharing are static,
paper-based and designed around legal frame-
works and regulation. They are also specific to in-
dividual research studies and have to be repeated
for subsequent studies. There is a growing aware-
ness that this is inadequate and future policies are
moving towards a more sophisticated form of con-
sent (e.g. the proposed EU General Data Protec-
tion Regulation (GDPR, 2016)). Dynamic con-
sent provides patients with a personalised inter-
face, placing them in control of how their health-
care data is used; data sharing preferences can
be stated and often they can view how their data
is being used by researchers (Kaye et al., 2015),
(Williams et al., 2015). Once consent has been
specified by patients, new tools and technologies
are required which enable their preferences to
be dynamically and automatically applied across
multiple clinical databases and research studies.
• Safe Havens - To control how electronic pa-
tient data is used by researchers, many healthcare
providers are making it accessible through Safe
Havens (i.e. it doesn’t leave an authorised envi-
ronment) (Caldicott, 2016). Safe Havens pull to-
gether data from multiple healthcare sources and
links made between the datasets whilst maintain-
ing patient confidentiality. Safe Havens require a
suite of software tools to: ensure security of the
centrally stored data (e.g. defend against cyber at-
tacks), enforce data access controls, and audit the
use of the patient data. Whilst basic tools have
been implemented, there is still potential for more
sophisticated software to support these activities.
• De-identification of Patient Data - Generally,
there is public support for the sharing of de-
identified data for research purposes. National
and international guidelines specify methods for
de-identification and can include the removal or
generalisation of certain attributes. Experts can
also be asked to identify attributes with an associ-
ated risk leading to patient identification. As re-
moval of data can lead to a lack of quality of the
dataset overall, there is a balance to be struck be-
tween usability and patient confidentiality. This is
a non-trivial optimisation problem which comput-
ing and artificial intelligence fields are well placed
to contribute towards workable solutions.
• Re-identification of Patient Data - Even when
patient data has been de-identified, there is still a
possibility that it can be re-identified through the
use of other, publicly available, datasets. This is
likely to be a growing concern, especially with
initiatives to make more data available and ma-
chine readable (e.g. Semantic Web). Some so-
lutions to reduce the chances of this happening
include: removal of high risk variables from a
dataset (e.g. features which are available in multi-
ple documents and publicly available); and gener-
alisation of patient data into ‘bins’ of data (e.g.
values are generalised over 5 patients). Again,
computing and artificial intelligence fields are
well placed to develop tools which enable the au-
tomatic identification of high risk attributes.
6 CONCLUSION
The health informatics community has an important
role to play in the development of novel technology
and algorithms to enable advances in clinical knowl-
edge and the quality of patient care. This type of re-
search requires access to sufficient volumes of patient
data which raises important issues by the general pub-
lic regarding ethics, trust and security of patient data,
especially if private companies are involved in the re-
search activities. Our position is that, despite these
concerns, it is necessary for private companies, re-
search institutions and healthcare providers to work
together to successfully transition technology projects
from research to real-world environments. However,
it is vital that patient confidentiality is maintained dur-
ing all stages of development. There is a role for
policy makers to ensure that existing legislation and
procedures are adequate for a fast moving technology
industry and that there is clear accountability. Addi-
tionally, there needs to be greater public engagement
on health informatics projects and open communica-
tion regarding the potential use of their data.
ACKNOWLEDGEMENTS
The CHART-ADAPT project is a collaboration be-
tween the University of Glasgow, Aridhia, NHS
Greater Glasgow & Clyde, and Philips Healthcare.
It has been co-funded by Innovate UK (ref:102113).
Approval for the CHART-ADAPT work was granted
by the West of Scotland Research Ethics Committee
(REC ref: 15/WS/0222) and local Caldicott Guardian
approval has been provided. Approval for the sur-
vey (section 4) was provided by MVLS, University of
Sharing of Big Data in Healthcare: Public Opinion, Trust, and Privacy Considerations for Health Informatics Researchers
467

Glasgow. The CHART-ADAPT project we would like
to acknowledge the staff and patients of the Neuroin-
tensive care unit, Neurosciences Institute, Glasgow.
REFERENCES
A.Ferguson (2012). The evolution of confidentiality in the
united kingdom and the west. AMA Journal of Ethics,
14(9):738–742.
Aitken, M., Cunningham-Burley, S., and Pagliari, C.
(2016a). Moving from trust to trustworthiness: Ex-
periences of public engagement in the scottish health
informatics programme. Science and Public Policy,
43(5):713–723.
Aitken, M., de St Jorre, J., Pagliari, C., Jepson, R., and
Cunningham-Burley, S. (2016b). Public responses to
the sharing and linkage of health data for research pur-
poses: a systematic review and thematic synthesis of
qualitative studies. BMC Medical Ethics, 17(73).
Apache a (2016). Apache Spark. https://spark.apache.org/.
Accessed: Nov 2016.
Apache b (2016). Apache Hadoop.
https://hadoop.apache.org/. Accessed: Nov 2016.
BMA (2016). Secondary Uses of Data, Public Workshop.
https://www.bma.org.uk/collective-voice/policy-
and-research/ethics/secondary-uses-of-data/public-
workshop. Accessed: Nov 2016.
Caldicott (2016). Information: To share or not to
share? The Information Governance Review.
https://www.gov.uk/government/uploads/system/
uploads /attachment data/file/192572/2900774
InfoGovernance accv2.pdf. Accessed: Nov 2016.
CHART-ADAPT (2016). CHART-ADAPT.
http://www.chartadapt.org. Accessed: Nov 2016.
DeepMind a (2016). DeepMind Acute Kidney In-
jury. Royal Free London. Google DeepMind:
Q&A. https://www.royalfree.nhs.uk/news-
media/news/google-deepmind-qa/. Accessed:
Nov 2016.
DeepMind b (2016). DeepMind Moorfields Eye
Hospital. Moorfields announces research partner-
ship. http://www.moorfields.nhs.uk/news/moorfields-
announces-research-partnership. Accessed: Nov
2016.
Fillmore, C., Braye, C., and Kawamoto, K. (2013). System-
atic review of clinical decision support interventions
with potential for inpatient cost reduction. BMC Med
Inform Decis Mak, 13(135).
Focus Groups, Stevenson, F., Lloyd, N., Harrington, L., and
Wallace, P. (2013). Use of electronic patient records
for research: views of patients and staff in general
practice. Fam Pract, 30(2):227–23.
GDPR (2016). GDPR: Regulation (EU)
2016/679. http://ec.europa.eu/justice/data-
protection/reform/files/regulation oj en.pdf. Ac-
cessed: Nov 2016.
IBM a (2016). IBM Big Data. Extracting busi-
ness value from the 4 V’s of big data.
http://www.ibmbigdatahub.com/infographic/extracting
-business-value-4-vs-big-data. Accessed: Nov 2016.
IBM b (2016). The 4 V’s of big data.
http://www.ibmbigdatahub.com/infographic/four-
vs-big-data. Accessed: Nov 2016.
IBM c (2016). IBM’s Watson supercomputer to speed up
cancer care. http://www.bbc.co.uk/news/technology-
32607688. Accessed: Nov 2016.
Jaspers, M., Smeulers, M., Vermeulen, H., and Peute, L.
(2011). Effects of clinical decision-support systems
on practitioner performance and patient outcomes: a
synthesis of high-quality systematic review findings.
J Am Med Inform Assoc, 18(3):327–34.
Kaye, J., Whitley, E., Lund, D., Morrison, M., Teare, H.,
and Melham, K. (2015). Dynamic consent: a patient
interface for twenty-first century research networks.
Eur J Hum Genet, 23(2):141–6.
Kinsella, J., Hawthorne, C., Shaw, M., Piper, I., Healthcare,
P., Aridhia, and L.Moss (2017). Public perception of
the collection and use of critical care patient data be-
yond treatment: a pilot study. In Proceedings of the
Society of Critical Care Medicine Congress (SCCM).
SCCM.
Kitchen, R. and McArdle, G. (2016). What makes big data,
big data? exploring the ontological characteristics of
26 datasets. Big Data & Society, Jan-June 2016(3):1–
10.
New Scientist (2016). Revealed: Google AI
has access to huge haul of NHS pa-
tient data. New Scientist 2016 Apr 29.
https://www.newscientist.com/article/\2086454-
revealed-google-\ai-has-access-to-\huge-haul-of-
nhs-patient-data/. Accessed: Nov 2016.
Presser, L., Hruskova, M., Rowbottom, H., and Kancir, J.
(2015). Care.data and access to uk health records:
patient privacy and public trust. Technology Science,
2015081103(Aug 11).
Riordan, F., Papoutsi, C., Reed, J., Marston, C., Bell, D.,
and Majeed, A. (2015). Patient and public attitudes
towards informed consent models and levels of aware-
ness of electronic health records in the uk. Int J Med
Inform, 84(4):237–347.
Scala (2016). Scala Programming Language.
http://www.scala-lang.org/. Accessed: Nov 2016.
van Staa, T.-P., Goldacre, B., Buchan, I., and Smeeth, L.
(2016). Big health data: the need to earn public trust.
BMJ, 354:i3636.
Williams, H., Spencer, K., Sanders, K., Lund, D., Whitley,
E., Kaye, J., and Dixon, W. (2015). Dynamic consent:
A possible solution to improve patient confidence and
trust in how electronic patient records are used in med-
ical research. JMIR Med Inform, 3(1).
HEALTHINF 2017 - 10th International Conference on Health Informatics
468
