
UV Pulsed Laser Irradiation Effect on Spectral Properties of
Borosilicate and Phosphate Glasses with CuCl Nanocrystals
Anastasiia Babkina, Ksenya Trots, Elena Kolobkova and Nikolay Nikonorov
Department of Optical Information Technologies and Materials, ITMO University,
49 Kronverksky Pr., St. Petersburg, Russia
Keywords: Phosphate Glass, Borosilicate Glass, Copper Chloride, Nanocrystals, Photochromism, Exciton Absorption.
Abstract: The results of the study of the pulsed UV laser radiation effect on the spectral properties of the borosilicate
and the phosphate glasses doped with the copper chloride nanocrystals with the mean size of 26-70 Å are
discussed. The changes of the exciton absorption spectra of the CuCl nanocrystals with various mean sizes
induced by different duration of the laser exposure are studied. The effect of the phosphate glass
transmission reduction in the visible region upon pulsed UV laser irradiation is obtained for the first time.
The nature of the transmission reduction is discussed. The assumption is made that the transmission
reduction is carried out through the formation of the color centers consisted of the Cu
n
(n>13) clusters which
have the absorption bands in the visible region. In conclusion the presence of the irreversible
photochromism in the phosphate glass is stated.
1 INTRODUCTION
In the semiconductor crystal field the study of
properties of the copper chloride crystals is of a
great interest for the development of photonics
nowadays. Copper chloride is a wide-gap
semiconductor with allowed direct interband
electron transitions. Macrocrystals of CuCl have
been well studied (Cardona, 1963) and are known to
demonstrate the intense exciton absorption at the
edge of the band gap and the negative spin-orbit
splitting (Goldmann 1977).
Borosilicate (BS) glasses, activated by the CuCl
nanocrystals with the mean size of more than 70 Å,
are known to exhibit reversible photochromism
(Dotsenko et al., 1998): continuous exposure by
ultraviolet radiation leads to the reduction of the
glass transmission in the visible region, when the
activating irradiation is switched off the glass
transmission returns back to its initial level.
Composite materials with the reversible
photochromism are widely used for the protection of
the human eye and the detectors of the
optoelectronic systems from UV and stray visible
radiation. In case of the nucleation of the CuCl
crystals having the mean size of over 100 Å the
glass transmission reduction under UV irradiation
occurs due to the presence of the surface plasmon
resonance absorption band of metallic Cu
0
nanoparticles centred at 560-580 nm (Sheng et al.,
2009; Morse, 1981). With the presence of the CuCl
crystals having the mean size between 70 and 100 Å
the transmission of the silicate glass during UV
irradiation is reduced due to the nucleation of the
Cu
0
n
clusters (n> 13), the absorption bands of which
occupy the region of 360-460 nm (Vázquez-
Vázquez et al., 2009). It is noteworthy that the
copper nanoparticles are formed only in the presence
of the interface between the nanocrystalline phase
(NCP) and the “vacuum pore” (Golubkov, 1986).
The certain interface appears only when the large
size droplets of the copper halide phase at
temperatures above glass transition temperature and,
consequently, the large size crystals at room
temperature exist. When the size of the phase droplet
is small, the difference between the volumes of
liquid and crystal phases will be too small for the
formation the vacuum pore of full value (Golubkov,
1982), on the boundary of which the nucleation of
the copper particles or clusters can take place under
the UV irradiation.
Phosphate glasses doped with CuCl nanocrystals,
which have recently been discussed for the first time
in works (Shirshnev et al., 2015; Babkina et al.,
2015), show the effect of nonlinear optical limiting
due to the two-photon absorption under the pulsed
298
Babkina A., Trots K., Kolobkova E. and Nikonorov N.
UV Pulsed Laser Irradiation Effect on Spectral Properties of Borosilicate and Phosphate Glasses with CuCl Nanocrystals.
DOI: 10.5220/0006221102980303
In Proceedings of the 5th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2017), pages 298-303
ISBN: 978-989-758-223-3
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

laser irradiation of 532 nm. Hitherto there are no
papers dedicated to the interaction of these glasses
and the laser irradiation of UV range.
Therefore, in the present paper we carry out the
comparison study on the pulsed UV laser irradiation
effect on the spectral properties of the borosilicate
and the phosphate glasses activated by the CuCl
nanocrystals.
2 MATERIALS AND METHODS
The following glass systems are used as the objects
of this study: borosilicate and phosphate. The
composition of the BS glass is similar to the glass
matrix described in (Golubkov, 1982) and comprises
: 56.3 SiO
2
– 28.3 B
2
O
3
– 12.1 Na
2
O – 3.3 Al
2
O
3
–
1.2 Cu
2
O – 1.2 P
2
O
5
– 1.7 Cl
-
- 0.7 F
-
(wt. %). The
phosphate glass of the following composition:
45 P
2
O
5
–19 BaO - 12 Na
2
O – 7 Al
2
O
3
– 8 F
-
-
1Cu
2
O - is used as a constant with a variable value
of Cl
-
from 8 to 11.6 (wt. %). All glasses were
prepared in the high temperature furnace Gmp
(Gero) using a platinum and a carbon crucible for
the BS and the phosphate glass respectively and a
platinum stirrer for the glass melt homogenization.
After the synthesis the phosphate glass was
quenched to room temperature and the BS glass was
annealed in a stepwise regime. The final glass
chemical composition determination was obtained
by X-ray fluorescence spectrometer ARL
PERFORM'X 4200 (Thermo Scientific). Glass
transition temperatures were determined by
differential scanning calorimeter STA 449F1 Jupiter
(Netzsch) and were found to be 757 K and 673 K for
the BS and the phosphate glass respectively.
The nanocrystalline phase was precipitated in the
BS glass bulk by isothermal treating the samples at
temperatures exceeding glass transition temperature
and subsequent quenching to room temperature.
Because the concentrations of both copper and
halogen exceeded the solubility limit for the matrix,
the system obtained might be considered to be the
supersaturated solid solution. During the high-
temperature heat treatment of this glass, the phase
separation of the supersaturated solid solution and
subsequent fluctuation nucleation of a new phase
occurred (Onushchenko, 1996; Ekimov, 1996).
The
usage of the hydrochloride acid during the phosphate
glass synthesis created the hard reducing conditions
so that the nanocrystalline phase nucleation was
conducted during quenching after it. In former case
the average size and concentration of the
nanocrystalline phase were defined by heat
treatment options, in latter one they were derived
from the hydrochloride acid concentration.
The glass samples irradiation was carried out by
the third harmonic of the Nd
3+
:YAG laser with a
wavelength of 355 nm, a pulse length of 9 ns, a peak
power of 13.2 MW/cm
2
and frequency of 10 Hz. The
absorption spectra of the samples were recorded at
room temperature before and after the irradiation
process. The deuterium lamp AvaLight-DH-S-BAL
(Avantes) was used as a light source and the fiber
optic spectrometer AvaSpec-2048L (Avantes) as a
detector.
3 RESULTS
Fig. 1 shows the absorption spectra of the initial BS
glass, the BS glass with the CuCl nanocrystals,
precipitated after the heat treatment at 823 K, and
the BS glass with the CuCl nanocrystals after
irradiation of various duration. The BS glass heat
treatment at temperature of 823 K leads to the
precipitation of the CuCl nanocrystals with the mean
size of 70 Å. The strong exciton absorption bands
occur near the edge of the crystal band gap after the
heat treatment at temperatures exceeding glass
transition temperature. The mean crystal size
calculations are produced from the optical spectra
according to the method described in (Efros, 1982).
According to the work (Dotsenko et al., 1998) such
treatment leads to the arising of the glass sensitivity
to the UV radiation, i.e. the photochromic effect
occurrence. UV laser irradiation of the BS glass
promotes the occurrence of the strong absorption
band centered at 340 nm (3.65 eV) and the weak
absorption band at 450 nm (2.76 eV). The increase
in the duration of the exposure leads to the increase
in the intensity of the laser-induced absorption and
the CuCl exciton absorption bands. The difference in
the absorption intensity between the non-radiated
and irradiated during 10 minutes the BS glass
samples at the wavelength of 340 nm is 35.5 cm
-1
, at
367 nm (maximum of Z
1,2
CuCl absorption band) is
18 cm
-1
and at 450 nm is 5.7 cm
-1
. The transmission
decrease in the visible region is about 53%. In case
of the precipitation of the CuCl nanocrystals having
the mean size 26 Å the BS glass does not become
photochromic, and the radiation-induced increase of
absorption at the same wavelengths is about two
times less than in the first sample.
UV Pulsed Laser Irradiation Effect on Spectral Properties of Borosilicate and Phosphate Glasses with CuCl Nanocrystals
299
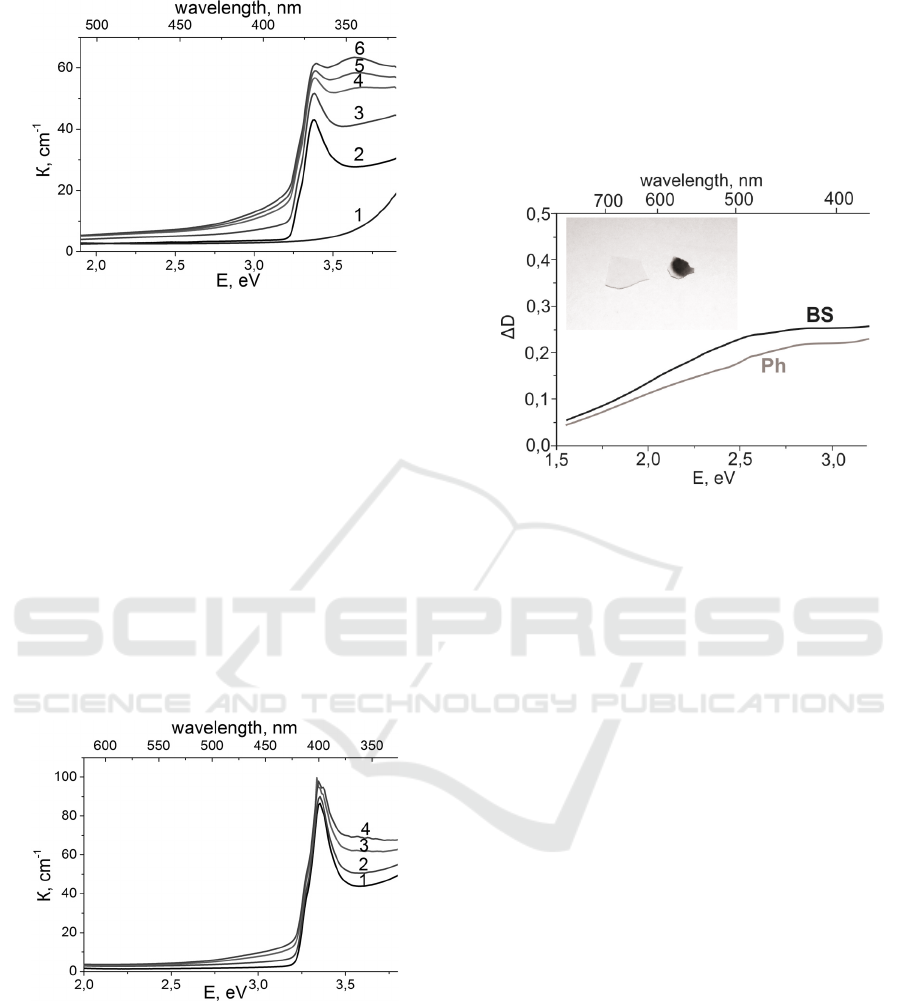
Figure 1: The absorption spectra of the BS glass before
heat treatment (1), after heat treatment and before
irradiation (2) and after irradiation for 100 sec (3), 300 sec
(4), 400 sec (5), 600 sec (6).
Fig. 2 shows the absorption spectra of phosphate
glass doped with CuCl nanocrystals with the mean
size of 70 Å before and after irradiation by pulsed
UV laser. The irradiation for 10 minutes leads to the
absorption increment at 340 nm (3.65 eV) and
450 nm (2.76 eV) of 23.87 and 4.25 cm
-1
,
respectively. The transmission decrease in the
visible region after irradiation is turned out to be
45%. If the CuCl nanocrystals having the mean size
of 34 Å have been precipitated in the phosphate
glass the radiation-induced increase of absorption in
the visible and UV regions is almost the same as in
case of the bigger nanocrystals.
Figure 2: The absorption spectra of the phosphate glass
before irradiation (1) and after irradiation for 100 sec (2),
300 sec (3), 600 sec (4).
The additional absorption spectra of the color
centers formed by 20-minutes UV-laser irradiation
of the BS and the phosphate glasses are shown in
Fig.3. General view of the additional absorption
band of different glasses is almost identical.
Figure 4 demonstrates the time dependence of
the optical density at 500 nm of the glass samples
under study during I-irradiation, II-relaxation (with
switched off excitation) and III-re-irradiation. The
samples were chosen so that the level of their initial
optical density is the same. The formed color centers
show the temporal stability in the phosphate glass,
while in the BS glass the laser-induced absorption
relaxes for 5 days.
Figure 3: The additional absorption spectra of the color
centers formed in glasses with CuCl NC after pulsed laser
irradiation. The inset: photos of the non-radiated (left) and
the irradiated (right) phosphate glass.
4 DISCUSSION
Despite the difference in the glass matrices
compositions, the location of the radiation-induced
absorption bands is the same. As to the reasons
responsible for this situation, some tentative
assumptions can only be proposed for discussing.
According to the works (El-Batal, 2008; ElBatal
et al., 2013; Barkatt et al., 1981; Bishay, 1970;
Möncke et al., 2014; Narayanan, 2015b; Narayanan,
2015a) the phosphate glass structure comprises
[PO
4
] tetrahedra. In the case of the pure amorphous
phosphorus (V) oxide the [PO
4
] groups are
connected to the neighbour units by three out of the
four oxygen atoms, and the latter one is connected to
the phosphorus by the double bond (the terminal
oxygen). Although the phosphate glass structure
changes take place during the introduction of the
alkaline oxides, the phosphorus remains the four
coordinated state in the entire range of compositions
from the pure P
2
O
5
to the orthophosphate MPO
4
saturated by alkaline oxides. When the alkaline
oxides are added to the amorphous P
2
O
5
the
phosphate structural units change from Q
3
to Q
2
,
then to Q
1
, and, finally, to Q
0
, together with a molar
ratio of the alkali metal oxide to the phosphorus
oxide M
2
O/P
2
O
5
=R, where R varies from 0 to 3
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
300
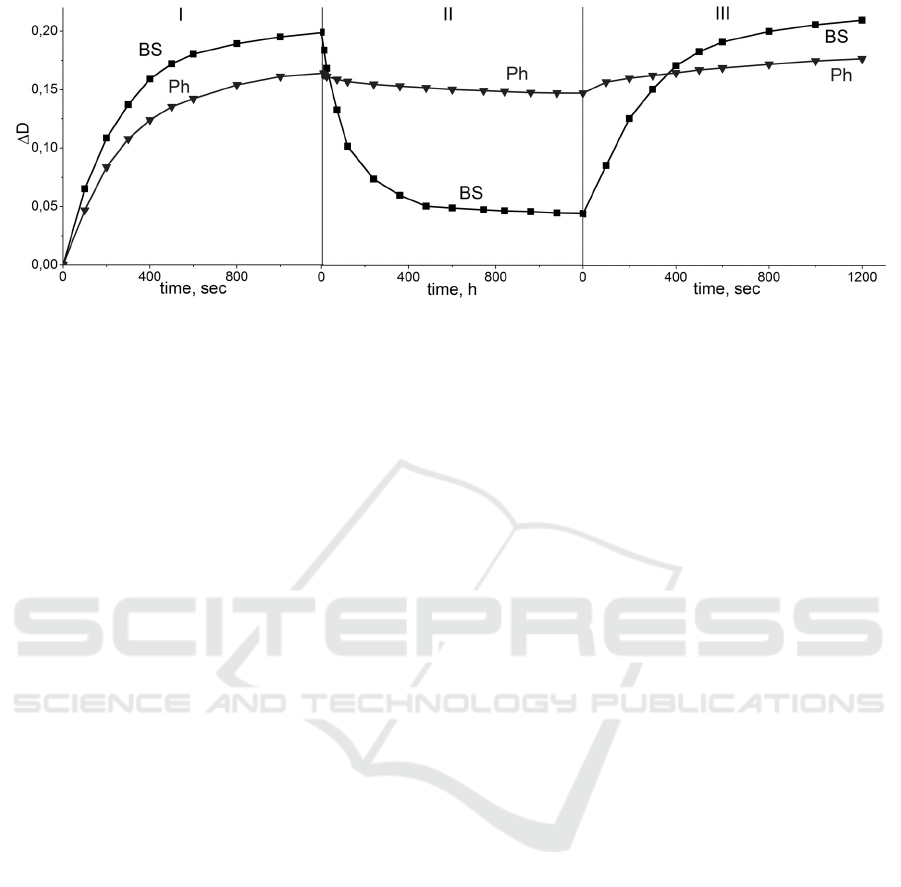
Figure 4: Optical density time kinetics of the glasses during I-irradiation, II-subsequent relaxation and III-second
irradiation.
(Narayanan and Shashikala, 2015a). A similar
transformation of the structure with the alkali
addition occurs in the silicate glass. With the
addition of M
2
O a number of the terminal oxygen
remains, while the number of non-bridging oxygen
changes. At first, the addition of M
2
O or MO (e.g.,
Na
2
O or CaO) leads to the transformation of the
three-dimensional amorphous P
2
O
5
into the linear
phosphate chain. These structures lead to the
destruction of the P-O-P bonds and the formation of
the terminal oxygen atoms. Due to a decrease in the
average length of the phosphate chain, the increasing
concentration of Cu
2
O enhances the covalent
character of the P-O-O bonds thus leading to the
glass depolymerization. The depolymerization of the
ring and chain structures with the addition of the
monovalent metal oxides occurs also in the silicate
glasses.
In several sources (Ruller, 1991; Tsai et al.,
1990) have been shown that the exposure by the
ionizing radiation or by optical radiation with high
energy leads to the compression of the amorphous
silica due to the breaking of the Si-O-Si bonds and
the formation of the ≡Si type defects (E’ centers).
The strained Si-O bonds in the rings consisting of 6
or more SiO
4
tetrahedra capture the radiolytic
charge, which breaks them and allows the ring
structure to relax and become more compact, for
example, to be transformed into the ring with a small
number of the tetrahedra and a more dense packing.
X-ray irradiation of the multicomponent silicate
glass (Tsai et al., 1989; Tsai et al., 1987) promotes
the formation of the oxygen hole centers, which
have the absorption in the region of 440-460 nm
(Bishay, 1970). In this study the laser radiation with
energy lower than the optical band gap of the
material is used, therefore, the change of the glass
structure, namely the Si-O bonds reflow, is not
plausible. On the other hand, if a transmittance
reduction of the BS glass depends only on the
concentration of the radiation-induced E' centers, the
connection of this effect with the mean crystal size
remains unclear.
According to (El-Batal, 2008; ElBatal et al.,
2013) the high-energy irradiation of the phosphate
glasses results in the formation of several types of
the glass network defects, namely: the oxygen
vacancy in the в [PO
4
] tetrahedra with two trapped
electrons; the trapped electron centre; the trapped
hole centre near non-bridging oxygen; the trapped
hole centre near the monovalent metal ion covalently
bonded with oxygen, - which have the absorption
bands centred at 200, 225, 420 and 540 nm,
respectively.
Due to a similar behavior of the monovalent
copper ions in the structure of different glasses, the
similar nature of the defects initiated by pulsed UV
irradiation can be assumed. As it has been
mentioned above, in all the glasses under study a
possibility of the formation of the defects such as
trapped electrons and holes (Bishay, 1970), and free
electrons and holes does exist. The defects in a
similar environment, for example, near the copper
ions and the non-bridging oxygen, would have the
absorption bands in the same range. The free
electrons created due to the inflow of the additional
energy from the laser beam cause the reduction of
the divalent copper ions to the monovalent state and
the monovalent copper ions to the copper atoms.
Assuming the high localization of the copper atoms
the opportunity of the Cu
n
molecular clusters
formation arises. The molecular copper clusters with
n <10 demonstrate luminescence under the
excitation by UV emission (Vázquez-Vázquez et al.,
2009). In this study the irradiation of samples by
340 nm does not initiate any emission. However, the
UV Pulsed Laser Irradiation Effect on Spectral Properties of Borosilicate and Phosphate Glasses with CuCl Nanocrystals
301

Stokes shift between the excitation and the emission
of the Cu
n
(n=3-5) molecular clusters is about 4000-
5000 cm
-1
, so that the initiated luminescence bands
would be located in the region of the exciton
absorption of the CuCl nanocrystals. The excitation
by longer wavelengths has not yielded the expected
emission either. This is why, the nature of the color
centers can be attributed to two possible situations:
first, the glass network defects, originated from the
powerful laser irradiation, and, second, the Cu
n
(n>13) clusters, which demonstrate no fluorescence,
but can be characterized by the absorption bands in
the visible region. The phosphate glass network
defects mentioned above also do not reveal the
fluorescent properties.
Let us consider a model of the nanocrystalline
phase proposed in the works (Golubkov, 1998;
Golubkov, 1982). During the thermal treatment of
the glass doped with the copper and chlorine ions at
temperatures above glass transition temperature due
to the presence of spatially fluctuations in the
chemical composition of the glass the regions with
an increased content of the future nanocrystalline
phase components are formed. The presence of the
inhibitor defects in the glass allows the
concentration increase of such areas. In regard of the
multi-component glass the composition of these
areas is not uniform: besides the copper and chlorine
molecular compounds it may include some
components of the glass matrix. During the cooling
process the thermal contraction of the copper halide
droplet phase and the glass matrix does occur in
accordance with their linear thermal expansion
coefficients. In the BS glass the linear thermal
expansion coefficient of the glass matrix is three
times smaller than the one of the CuCl (Golubkov et
al., 2012; Dotsenko et al., 1998). Therefore, the
contraction of the nanocrystalline phase goes faster
than the contraction of the matrix, thus in the
interlayer between the nanocrystalline phase and the
glass matrix the “vacuum pore” is formed, whose
presence has been shown in the works (Golubkov,
1986). The mechanism of the radiation-induced
darkening of the BS glass is the following. UV
irradiation leads to ionization on the surface of the
CuCl nanocrystalline phase, to the occurrence of the
so-called "halide gas" consisting of Cl
2
0
in the
“vacuum pore” and to the formation of the metallic
copper film on the nanocrystalline phase surface
with the absorption in the visible region.
The formation of the areas with high
concentration of the components of the future
nanocrystalline phase in phosphate glasses occurs
during the glass synthesis. It is known (Ehrt, 1992)
that the introduction of a large amount of fluorine
(as in our case) to the phosphate glass leads to the
incorporation of the fluorine into the glass structure
and, therefore, to the depolymerization of the
phosphate chains and the formation of the defect end
structures, next to which the components of the
future nanocrystalline phase can be accumulated.
The fluorine content in all phosphate glasses under
study was the same, so, the probability of the
formation of these defects was equal. The thermal
expansion coefficients of the phosphate matrix and
the CuCl are approximately equal, therefore, during
quenching after the synthesis the matrix and the
nanocrystalline phase are compressed in the same
way. The nanocrystalline phase droplets detachment
from the glass does not seem plausible, so, it is
impossible to talk about the “vacuum pore”
formation. Surface tension between the copper
halide droplet phase and the matrix always
compresses drop so that a part of its composition
falls into the matrix. As a result the matrix is
enriched with nanocrystalline phase components
around the droplets (Golubkov, 1998). The copper
ions trapped in this transition layer would be highly
localized. This is a favorable condition for the
creation of the Cu
n
(n>13) molecular clusters.
The difference in sensitivity to UV radiation
between the BS and the phosphate glasses can be
assigned to, firstly, the presence of a clear interface
between the matrix and the nanocrystalline phase in
the BS glasses providing greater localization of the
copper ions in the transition layer unlike the
phosphate glasses, where the interface does not
appear; secondly, as the nanocrystalline phase in the
phosphate glasses nucleates during quenching after
the glass synthesis a large amount of the copper ions
is distributed in the matrix in the free form, during
the laser irradiation they can be reduced to the
atomic form, but they concentration is too small to
form the particles or the molecular clusters.
5 CONCLUSIONS
As a conclusion, it can be stated that the irreversible
photochromism occurs in the phosphate glass
activated by CuCl nanocrystals. The differences in
sensitivity to UV irradiation between the borosilicate
and the phosphate glasses are mostly associated with
the methods of the nanocrystalline phase formation
in different glass matrices and with the difference in
thermal expansion coefficients of the glasses under
study. Materials possessing the irreversible
photochromic effect, unlike reversible one, can be
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
302

used for the amplitude image recording and for
storing information in the form of the Bragg
gratings.
ACKNOWLEDGEMENTS
Research was funded by Russian Science
Foundation (Agreement #14-23-00136).
REFERENCES
Babkina, A.N. et al., 2015. Spectral properties of copper
halide nanocrystals in glasses of fluorine-phosphate
matrix. Optics and Spectroscopy, 119(2), pp.243–247.
Available at: http://link.springer.com/10.1134/
S0030400X15080032.
Barkatt, A., Angell, C.A. & Miller, J.R., 1981. Visible
Spectroscopy of Irradiated High-Alkali Borate and
Mixed-Alkali Phosphate Glasses. Journal of American
Ceramic Society, 64(3), pp.158–162.
Bishay, A., 1970. Radiation Induced Color Centers in
Multicomponent Glasses. J. of Non-Crystalline Solids,
3, pp.54–114.
Cardona, M., 1963. Optical Properties of the Silver and
Cuprous Halides. Physical Review, 129(1), pp.69–78.
Dotsenko, A. V., Glebov, L.B. & Tsekhomsky, V.A.,
1998. Physics and Chemistry of Photochromic
Glasses, New York: CRC Press.
Efros, A.L. & Efros, A.L., 1982. Interband absorption of
light in a semiconductor sphere. Soviet physics.
Semiconductors, 16(7).
Ehrt, D., 1992. Structure and properties of fluoride
phosphate glasses. Proc. of SPIE, 1761, pp.213–222.
Ekimov, A., 1996. Growth and optical properties of
semiconductor nanocrystals in a glass matrix. Journal
of Luminescence, 70(1–6), pp.1–20. Available at:
http://www.sciencedirect.com/science/article/pii/0022
231396000403.
El-Batal, F.H., 2008. Gamma ray interaction with copper-
doped sodium phosphate glasses. Journal of Materials
Science, 43(3), pp.1070–1079.
ElBatal, H.A. et al., 2013. Gamma rays interaction with
copper doped lithium phosphate glasses. Journal of
Molecular Structure, 1054–1055, pp.57–64. Available
at: http://linkinghub.elsevier.com/retrieve/pii/
S0022286013007977.
Goldmann, A., 1977. Band Structure and Optical
Properties of Tetrahedrally Coordinated Cu- and Ag-
Halides. Phys. Stat. Sol (b), 81(9), pp.9–47.
Golubkov, V.V. et al., 2012. Precipitation of nanosized
crystals CuBr and CuCl in potassium aluminoborate
glasses. Glass Physics and Chemistry, 38(3).
Golubkov, V.V. & Tsekhomskii, V.A., 1998. Composition
and structure of copper halide phase in sodium and
potassium aluminoborosilicate glasses. Glass Physics
and Chemistry, 24(1).
Golubkov, V.V. & Tsekhomskii, V.A., 1982. Phase
changes in Copper Halide photochromic glasses. The
Soviet journal of glass physics and chemistry, 8(4).
Golubkov, V.V. & Tsekhomskii, V.A., 1986. Role of
Sodium Chloride in the formation of a light-sensitive
phase in Copper Halide photochromic glass. The
Soviet journal of glass physics and chemistry, 12(2).
Möncke, D. et al., 2014. Irradiation-induced defects in
ionic sulfophosphate glasses. Journal of Non-
Crystalline Solids, 383, pp.33–37. Available at:
http://dx.doi.org/10.1016/j.jnoncrysol.2013.04.029.
Morse, D.L., 1981. Copper halide containing
photochromic glasses. Inorganic Chemistry, 20(3),
pp.777–780. Available at: http://pubs.acs.org/doi/abs/
10.1021/ic50217a028.
Narayanan, M.K. & Shashikala, H.D., 2015a. Physical,
mechanical and structural properties of BaO–CaF2–
P2O5 glasses. Journal of Non-Crystalline Solids, 430,
pp.79–86. Available at: http://linkinghub.elsevier.com/
retrieve/pii/S0022309315302106.
Narayanan, M.K. & Shashikala, H.D., 2015b. Thermal and
optical properties of BaO–CaF2–P2O5 glasses.
Journal of Non-Crystalline Solids, 422, pp.6–11.
Available at: http://linkinghub.elsevier.com/retrieve/
pii/S0022309315300119.
Onushchenko, A.A. & Petrovskii, G.T., 1996. Size effects
in phase transitions of semiconductor nanoparticles
embedded in glass. J. Non-Cryst. Sol., 196, pp.73–78.
Ruller, J.A. & Friebele, E.J., 1991. The effect of gamma-
irradiation on the density of various types of silica.
Journal of Non-Crystalline Solids, 136(1–2), pp.163–
172.
Sheng, J. et al., 2009. UV-light irradiation induced copper
nanoclusters in a silicate glass. International Journal
of Hydrogen Energy, 34(2), pp.1119–1122. Available
at: http://dx.doi.org/10.1016/j.ijhydene.2008.10.063.
Shirshnev, P. et al., 2015. Copper-containing potassium-
alumina-borate glass: Structure and nonlinear optical
properties correlation. PHOTOPTICS 2015 - 3rd
International Conference on Photonics, Optics and
Laser Technology, Proceedings, 1, pp.108–112.
Tsai, T.E. et al., 1989. Radiation-induced defect centers in
glass ceramics. Journal of Applied Physics, 65,
pp.507–514.
Tsai, T.E. et al., 1987. Radiation effects on a low-thermal-
expansion glass ceramic. Journal of Applied Physics,
62(8), p.3488. Available at: http://scitation.aip.org
/content/aip/journal/jap/62/8/10.1063/1.339272.
Tsai, T.E., Griscom, D.L. & Friebele, E.J., 1990. Si E’
CENTERS AND UV-INDUCED COMPACTION IN
HIGH PURITY SILICA. Nuclear Instruments and
Methods in Physics Research B, 46, pp.265–268.
Vázquez-Vázquez, C. et al., 2009. Synthesis of small
atomic copper clusters in microemulsions. Langmuir,
25(14), pp.8208–8216.
UV Pulsed Laser Irradiation Effect on Spectral Properties of Borosilicate and Phosphate Glasses with CuCl Nanocrystals
303
