
Real-time Display and In-vivo Optical-resolution Photoacoustic
Microscopy for Ophthalmic Imaging
Sang-Won Lee
1, 2, 3
, Heesung Kang
1
and Tea Geol Lee
1, 2
1
Center for Nano-Bio Measurement, Korea Research Institute of Standards and Science, Daejeon, Rep. of Korea
2
Department of Nano Science, University of Science and Technology, Daejeon, Rep. of Korea
3
Center for Nanosafety Metrology, Korea Research Institute of Standards and Science, Daejeon, Rep. of Korea
Keywords: Optical-resolution Photoacoustic Microscopy, Ophthalmic Imaging, Angiography.
Abstract: Photoacoustic imaging is a non-invasive imaging technology that can be combined with optical absorption
contrast and detection of acoustic wave for structural, functional, and molecular imaging. Especially, optical-
resolution photoacoustic microscopy (OR-PAM) can provide a high spatial resolution with a micron-scale. In
this study, we have developed laser-scanning OR-PAM, which could obtain in-vivo photoacoustic ophthalmic
angiography. For high speed image acquisition, we used a nanosecond pulsed laser with a 300 kHz-pulse
repetition rates. In addition, we carried out parallel signal processing using a graphics processing unit to enable
fast signal processing. Therefore, we successfully obtained maximum amplitude projection images of
microvasculature in anterior and posterior segments of mouse’s eye with real-time display of 0.98 fps.
1 INTRODUCTION
Most of the imaging modalities in ophthalmology,
such as fundus camera (Pomerantzeff et al., 1979),
slit-lamp, scanning laser ophthalmoscope (Webb and
Hughes, 1981), and optical coherence tomography
(Huang et al., 1991), are based on detection of
reflected light or single-backscattered light (Jiao et
al., 2010). These imaging techniques have advantages
that can provide anatomical and functional images for
noninvasively accurate diagnosis of ocular diseases.
Optical coherence tomography (OCT) cannot provide
only three-dimensional and structural images but also
label-free functional images such as Doppler (Chen et
al., 1997), polarization (Ren et al., 2002), and
angiography (Wang et al., 2007). Additionally,
fundus camera and scanning laser ophthalmoscope
(SLO) can image retinal vasculature with high
transverse resolution using contrast agents such as
fluorescein and indocyanine green (Song et al., 2013).
However, these optical imaging tools cannot support
information of the molecules with optical absorption
properties in ocular tissues.
Photoacoustic imaging combined with optical
absorption contrast and detection of acoustic wave
has been actively studied because photoacoustic
imaging can provide structural, functional, and
molecular images (Wang et al., 2003, Zhang et al.
2006, Kim et al., 2010). Especially, optical-resolution
photoacoustic microscopy (OR-PAM) can provide
microscopic images with high spatial resolution using
tightly focused laser spot with a micro-scale (Maslov
et al. 2008, Xie et al. 2009)
Recently, several groups have developed
ophthalmic photoacoustic microscopy (Jiao et al.,
2010, Hu et al., 2010, de la Zerda et al., 2010,
Silverman et al., 2010). Hu et al. and de la Zerda et al.
have deomnstrated ocular OR-PAM images with an
acquisition speed of a few hours owing to usages of a
low pulse repetition rate laser and mechanical
scanning (Hu et al., 2010, de la Zerda et al. 2010).
Silverman et al. could achieve the imaging speed with
2.7 s for volumentric acqusition (Silverman et al.,
2010). However, their image pixel size was limited to
be 256 256 pixels. The imaging speed in OR-PAM
depends on the pulse repetition rate of the laser and
an acquired image pixel size.
The higher acquisition speed for an ophthalmic
image is important to reduce motion artifacts by
breathing, heartbeat, and eye movements. In addition,
small image pixel size can cause an low image pixel
resolution at the large field of view (FOV) although
an optical resolution by a tightly focused beam size is
very small.
34
Lee S., Kang H. and Geol Lee T.
Real-time Display and In-vivo Optical-resolution Photoacoustic Microscopy for Ophthalmic Imaging.
DOI: 10.5220/0006154300340038
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 34-38
ISBN: 978-989-758-215-8
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
In our previous study, we have demonstrated
maximum amplitude projection (MAP) images of
blood vessels in a mouse’s ear from volumetric data
set with the data size of 736 500 500 points at 1.02
seconds (Kang et al., 2015). For high speed image
acquisition, we used a nanosecond pulsed laser with
a pulse repetition rate of 300 kHz and carried out
parallel signal processing with graphics processing
unit (GPU).
In this study, we modified and applied our high-
speed laser-scanning OR-PAM system to obtain
photoacoustic ophthalmic images. Therefore, we
could obtain MAP images of microvasculature in
anterior and postierior segments of mouse’s eye with
real-time display of 0.98 fps and large pixel size of
500 500 pxiels.
2 EXPERIMENTAL METHODS
2.1 Laser-Scanning OR-PAM
Figure 1 shows a schematic diagram of a laser-
scanning OR-PAM. This schematic was modified
from our previous study (Kang et al., 2015). We used
an Ytterbium-doped fiber laser (YLP-G10, IPG
Photonics Corp.) with 1-ns pulse width and 300-kHz
pulse repetition rates at 532 nm. Light from the laser
was delivered by an optical fiber and scanned by 2-D
galvanometer scanning mirror with silver coating
(GVSM002, Thorlabs Inc.) as shown in Fig. 1 (a). In
our previous study, collimated light was scanned and
focused in inverse direction (bottom-to-top). In
addition, the acoustic waves was detected in direction
pass through a sample with optical path as shown in
Fig. 1(b). This schematic could obtain OR-PAM
images of blood vessels in mouse’s ear because
thickness of mouse’s ear is thin below 500 m.
However, in the eye, we could not apply acoustic
wave detection method in transmitted direction.
Therefore, we used a schematic of Fig. 1 (c) that the
acoustic waves occurred at the focal plane were
reflected by a thin glass with a tilting angle of 45 in
a water tank. Reflected acoustic wave detected by
ultrasound transducer. Finally, detected signals were
amplified by a pulser/receiver (5072PR, Olympus-
NDT) and digitally converted by a high-speed
digitizer (ATS9350, AlazarTech) at a sampling rate
of 250 MSamples/s.
For fast signal processing and real-time display,
we carried out parallel signal processing using GPU.
A graphics card (ASUS GTX780Ti, ASUSTeK
Computer Inc.) for GPU processing has 2880 stream
processors, a 7000 MHz memory clock and 3 GB of
RAM. To accelerate signal processing time and
display real-time OR-PAM images, we developed
custom software using Visual C++ of Visual Studio
2012 (Microsoft) and compute unified device
architecture (CUDA) technology (NVIDIA Corp.).
Figure 1: (a) Schematic diagram of laser-scanning OR-PAM for ophthalmic imaging, (b) previous our setup for light
illumination and acoustic wave detection, (c) modified setup for light illumination and acoustic wave detection.
Real-time Display and In-vivo Optical-resolution Photoacoustic Microscopy for Ophthalmic Imaging
35
2.2 Animal Preparation
We used BALB/c mice at 6 ~ 8 weeks of age as an
animal experiment model. The mice were housed
under standard conditions (room temperature 232°C,
humidity 50±10%) with a 12-h dark-light cycle and
were fed standard laboratory chow and water ad
libitum. The care, use, and interventions were
approved by the Korea Research Institute of
Bioscience and Biotechnology (KRIBB). Before
retinal imaging, mydriatic was applied to dilate the
pupil. During imaging, the anesthetized mice were
restrained in a customized holder.
3 RESULTS AND DISCUSSION
The volumetric data set was obtained at the laser
pulse repetition rate of 300 kHz and composed with
736 points per line, 500 lines per B-mode-frame, and
500 B-mode-frames per C-mode-frame. Therefore,
data size per volume was 351 MB (2 bytes 736
500 500). In previous study, when we carried out
signal processing without parallel processing using
GPU, the total processing time was approximately
31.2 s. However, we could accelerate the processing
time to 1.02 s using parallel processing using GPU.
To evaluate the transverse resolution of our OR-
PAM system, we obtained the MAP image of a USAF
1951 resolution target (Edmunds Optics) as a sample.
When an area 3 mm 3 mm was achieved as the
maximum FOV, the lines at group 5 and element 6
could be distinguished. Therefore, a lateral resolution
was measured to be approximately 17.5 m. In
addition, when the FOV was reduced to 1 mm 1 mm,
we could distinguish the lines at group 7 and element
1, corresponding to a lateral resolution of 7.8 m
(Kang et al., 2015). This difference of the lateral
resolution came results from changing an image pixel
resolution owing to sizes of FOV rather than a spot
size of focused beam.
Figure 2 shows the MAP image (a) and 3-D
volumetric rendering image (b) of microvasculature
in the iris of a BALB/c mouse. When the
microvasculature image was obtained, a focused
ultrasound transducer at the center frequency of 20
MHz (V317-SU-F1.00in-PTF, Olympus-NDT) was
used. Focused ultrasound transducer had a 6 dB
bandwidth of 42.21%. In the anterior segment, the
vessels in the iris can be seen clearly in the MAP
image as sown in Fig. 2 (a). In addition, the location
of the pupil was well displayed as a hole at the center
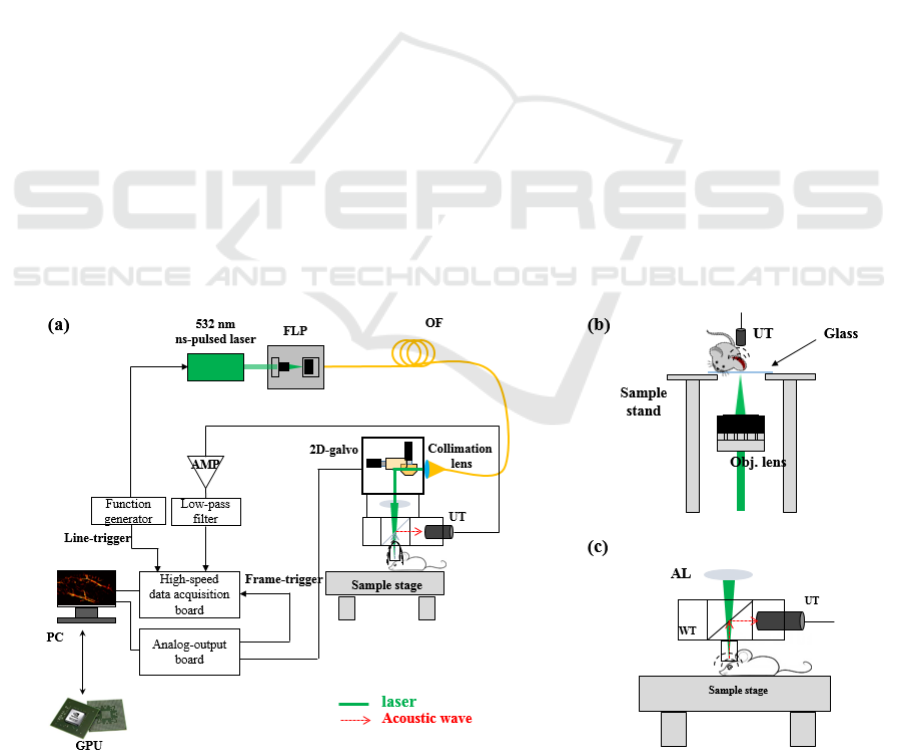
Figure 2: In-vivo photoacoustic ophthalmic angiography of
the iris microvasculature of a BALB/c mouse. (a) MAP
image and (b) 3-D rendering image.
of the iris. Figure 2 (b) was processed with Amira 6.1
(FEI Corp.).
Figure 3 shows MAP images of retinal
vasculature (a) and sclera choroidal vasculature (b),
respectively. We used an unfocused ultrasound
transducer at the center frequency of 15 MHz when
the vasculature images in the posterior segment of the
mouse’s eye was acquired. In previous paper, light
with a pulse energy of 500 nJ was illuminated to
image a posterior eye using focused laser beam (Wu
et al., 2014). In this study, we used a pulse energy of
approximately 600 nJ. As shown in Fig. 3 (a), we
could clearly observed that blood vessels in the retina
were gathered into optic disk area (red arrow). When
the focal position of light was shifted onto deeper area
and the incident angle of light was adjusted, we could
obtain the choroidal vascular (red arrows) image in
BIOIMAGING 2017 - 4th International Conference on Bioimaging
36
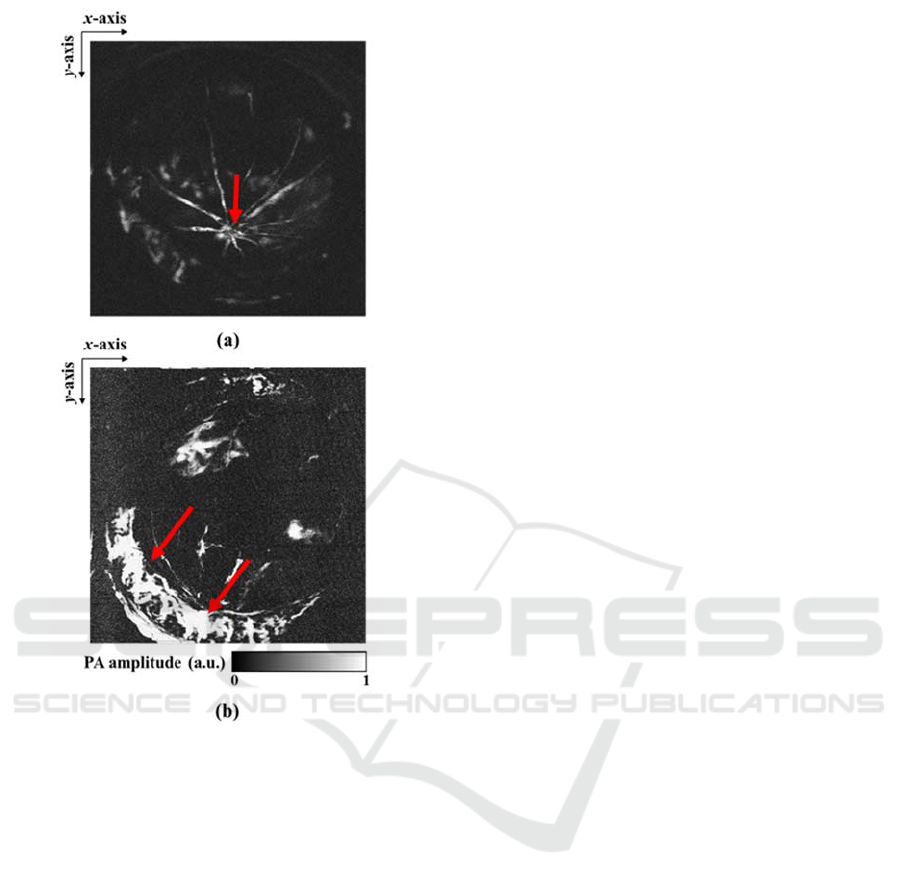
Figure 3: In-vivo photoacoustic ophthalmic angiography of
the posterior segment of a BLAB/c mouse. (a) Retinal
vasculature and (b) sclera choroidal vasculature.
mouse’s sclera as shown in Fig. 3 (b).
PAM have been studied as useful molecular
imaging tool with contrast agents in various medical
fields. PAM will be also used as a preclinical imaging
tool in ophthalmology for drug development and
diagnosis of disease targeted with specific receptors
such as the vascular endothelial growth factor using
nanoparticles or dyes. In addition, if PAM is
combined with various ophthalmic imaging tools
(OCT, fundus, and SLO), we can obtain structural,
functional, and molecular information.
4 CONCLUSIONS
In conclusion, we demonstrated real-time display
photoacoustic ophthalmic angiography using laser-
scanning OR-PAM at a mouse’s anterior and
posterior segment. We could display MAP images
with 500 500 pixels as volumetric images at 0.98
fps when we used a nanosecond pulse laser with 300-
kHz pulse repetition rates. In further study, we will
obtain molecular images to apply diagnosis of ocular
disease using bio-conjugated contrast agents, which
are based on optical absorbance such as nanoparticles
and dyes.
ACKNOWLEDGEMENTS
This work was supported by the “Development of
Platform Technology for Innovative Medical
Measurement Program (KRISS-2016-16011064)”
from the Korea Research Institute of Standards and
Science. It was also supported by grants from the
“Pioneer Research Center Program (2012-0009541)”
and the “Nano Material Technology Development
Program (2014M3A7B6020163)” through the
National Research Foundation (NRF), Rep. of Korea.
REFERENCES
Chen, Z., Milner, S. S., Wang, X., Malekafzali, A., van
Germent, M. J. C., & Nelson, J. S., 1997. Noninvasive
imaging of in vivo blood flow velocity using optical
Doppler tomography. Optics Letters, 22, 1119-21.
de la Zerda, A., Paulus, Y. M., Teed, R., Bodapati, S.,
Dollberg, Y., Khuri-Yakub, B. T., Blumenkranz, M. S.,
Moshfeghi, D. M. & Gambhir, S. S., 2010.
Photoacoustic ocuar imaging. Optics Letters, 35, 270-
2.
Hitenberger, C. K., Götzinger, E., Stricker, M., Pircher, M.,
& Fercher, A. F., 2001. Measurement and imaging of
birefringence and optic axis orientation by phase
resolved polarization sensitive optical coherence
tomography, Optics Express, 9, 780-90.
Hu, S. Rao, B., Maslov, K. & Wang, L. V., 2010. Label-
free photoacoustic ophthalmic angiography. Optics
Letters, 35, 1-3.
Huang, D., Swanson, E. A., Lin, C. P., Schuman, J. S.,
Stinson, W. G., Chang, W., Hee, M. R., Flotte, T.,
Gregory, K., Puliafito, C. A. & Fujimoto, J. G., 1991.
Optical coherence tomography. Science, 245, 1178-81.
Jiao, S., Jiang, M. Hu, J., Fawzi, A., Zhou, Q., Shung, K.
K., Pulifafito, C. A. & Zhang, H. F., 2010.
Photoacoustic ophthalmoscopy for in vivo retinal
imaging. Optics Express, 18, 3967-72.
Kang, H., Lee, S. W., Lee, E. S., Kim, S. H. & Lee, T. G.,
2015. Real-time GPU-accelerated processing and
volumetric display for wide-field laser-scanning
optical-resolution photoacoustic microscopy.
Biomedical Optics Express, 6, 4650-60.
Real-time Display and In-vivo Optical-resolution Photoacoustic Microscopy for Ophthalmic Imaging
37

Kim, C., Cho, E. C., Chen, J. Song, K. H., Au, L., Favazza,
C., Zhang, Q., Cobley, C. M., Gao, F., Xia, Y. & Wang,
L. V., 2010. In vivo molecular photoacoustic
tomography of melanomas targeted by bioconjugated
gold nanocages. ACS Nano, 4, 4559-64.
Maslov, K., Zhang, H. F., Hu, S. & Wang, L. V., 2008.
Optical-resolution photoacoustic microscopy for in
vivo imaging of single capillaries. Optics Letters, 33,
929-31.
Pomerantzeff, O., Webb, R. H. & Delori, F. C., 1979. Image
formation in fundus cameras. Invest Ophthalmol Vis Sci,
18, 630-7.
Silverman, R. H., Kong, F., Chen, Y. C., Lloyd, H. O., Kim,
H. H., Cannata, J. M., Shung K. K. & Coleman, D. J.,
2010. High-resolution photoacoustic imaging of ocular
tissues. Ultrasound in Medicine &Biology, 36, 733-42.
Song, W., Wei, Q., Feng, L. Sarthy, V. Jiao, S. Liu, X. &
Zhang, H. F., 2003. Multimodal photoacoustic
ophthalmoscopy in mouse, Journal of Biophotonics, 6,
505-12.
Wang, R. K., Jacques, S. L., Ma, Z., Hurst, S. Hanson, S. R.
& Gruber, A. Three dimensional optical angiography.
Optics Express, 15, 4083-97.
Wang, X., Pang, Y., Ku, G., Xie, X. Stoica, G. & Wang, L.
V., 2003. Noninvasive laser-induced photoacoustic
tomography for structural and functional in vivo
imaging of the brain. Nature Biotechnology, 21, 803-6.
Webb, R. H. & Hughes, G. W., 1981. Scanning laser
ophthalmoscope. IEEE Trans Biomed Eng, 28, 488-92.
Wu, N., Ye, S., Ren, Q. & Li, C., 2014. High-resolution
dual-modality photoacoustic ocular imaging. Optics
Letters, 39, 2451-4.
Xie, Z., Jiao, S., Zhang, H. F. & Puliafito, C. A., 2009.
Laser-scanning optical-resolution photoacoustic
microscopy. Optics Letters, 34, 1771-3.
Zhang, H. F., Maslov, K., Stoica, G. & Wang, L. V., 2006.
Functional photoacoustic microscopy for high-
resolution and noninvasive in vivo imaging. Nature
Biotechnology, 24, 848-51.
BIOIMAGING 2017 - 4th International Conference on Bioimaging
38
