
Dynamic Bayesian Network Modeling of Hippocampal Subfields
Connectivity with 7T fMRI: A Case Study
Fernando P. Santos
1
, Stephen F. Smagula
2
, Helmet Karim
3
, Tales S. Santini
3
, Howard J. Aizenstein
2
,
Tamer S. Ibrahim
3,4
and Carlos D. Maciel
1
1
Department of Electrical Engineering, S
˜
ao Carlos School of Engineering, University of S
˜
ao Paulo,
Av. Trabalhador Saocarlense, 400, 13561010, S
˜
ao Carlos, S
˜
ao Paulo, Brazil
2
Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine,
3811 O’Hara Street, 15213, Pittsburgh, Pennsylvania, U.S.A.
3
Department of Bioengineering, University of Pittsburgh, 3700 O’Hara Street, 15213, Pittsburgh, Pennsylvania, U.S.A.
4
Department of Radiology, University of Pittsburgh, 200 Lothrop Street, 15213, Pittsburgh, Pennsylvania, U.S.A
Keywords:
Dynamic Bayesian Networks, fMRI, Network Modelling, Hippocampus.
Abstract:
The development of high resolution structural and functional magnetic resonance imaging, along with the new
automatic segmentation procedures for identifying brain regions with high precision and level of detail, has
made possible new studies on functional connectivity in the medial temporal lobe and hippocampal subfields,
with important applications in the understanding of human memory and psychiatric disorders. Many previous
analyses using high resolution data have focused on undirected measures between these subfields. Our work
expands this by presenting Dynamic Bayesian Network (DBN) models as an useful tool for mapping directed
functional connectivity in the hippocampal subfields. Besides revealing directional connections, DBNs use
a model-free approach which also exclude indirect connections between nodes of a graph by means of con-
ditional probability distribution. They also relax the constraint of acyclicity imposed by traditional Bayesian
networks (BNs) by considering nodes at different time points through a Markovianity assumption. We apply
the GlobalMIT DBN learning algorithm to one subject with fMRI time-series obtained from three regions: the
cornu ammonis (CA), dentate gyrus (DG) and entorhinal cortex (ERC), and find an initial network structure,
which can be further expanded with the inclusion of new regions and analyzed with a group analysis method.
1 INTRODUCTION
Establishing functional connectivity networks in the
brain has become a major focus with the ad-
vance of neuroimaging techniques such as elec-
troencephalography (EEG), magnetoencephalogra-
phy (MEG), and functional magnetic resonance imag-
ing (fMRI) (Wang et al., 2015; Smith et al., 2011).
Specifically, fMRI presents advantages as it has high
spatial resolution, allowing connectivity studies to
focus on very specific parts of the brain including
deeper brain structures not accessible by MEG or
EEG. Therefore, one of the areas receiving attention
in recent years is the hippocampal sub-fields and more
specifically their connectivity. The hippocampus is a
structure located in the medial temporal lobe (MTL)
and has been associated with learning and memory
(Jeneson and Squire, 2012). Alterations in its struc-
ture and connectivity are shown to be associated with
several neurological and psychiatric disorders such
as Alzheimer’s disease, epilepsy, schizophrenia, and
others (Bartsch, 2012). Studies have been stressing
the importance of considering the hippocampal sub-
structures for a better understanding of its function
and pathologies, instead of considering it as a whole
(Maruszak and Thuret, 2015). However due to the
low resolution of the fMRI data, this type of analy-
sis was not possible on the hippocampus sub-fields,
and only recently, with the newest advances on ultra-
high field MRI with 7T scanners, higher-resolution
images are being obtained and analyzed(Duyn, 2012).
Moreover, advances in automatic segmentation proce-
dures also facilitate the extraction of regions of inter-
est (ROIs) and the usage of more data, since manual
segmentation may be very time consuming and prone
to errors (Wisse et al., 2016; Yushkevich et al., 2015).
Recently, studies have demonstrated functional subdi-
visions of the medial temporal lobe along the longitu-
178
P. Santos F., F. Smagula S., Karim H., S. Santini T., J. Aizenstein H., S. Ibrahim T. and D. Maciel C.
Dynamic Bayesian Network Modeling of Hippocampal Subfields Connectivity with 7T fMRI: A Case Study.
DOI: 10.5220/0006151601780184
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 178-184
ISBN: 978-989-758-212-7
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

dinal axis of the hippocampus (Libby et al., 2012), be-
tween hippocampal and para-hippocampal structures
(Libby et al., 2012; Das et al., 2013), and across sub-
fields (Lacy and Stark, 2012; de Flores et al., 2015).
Among the methods for brain connectiv-
ity,(Friston, 2011) has divided types of connectivity
into functional and effective connectivity, with the
first describing ”statistical dependencies among
remote neurophysiological events”, while the second
describes ”the influence that one neural system exerts
over another, either at a synaptic or population level”.
A spectrum of methods exist that fall between these
two approaches, with effective connectivity having
more complex and biologically informed models,
used for a confirmatory approach, while functional
connectivity presents simpler and more generic
methods, used for a exploratory approach (i.e.,
network discovery) (Valdes-Sosa et al., 2011). The
direction of the connections between nodes is also an
important factor — and in fact, recent work by (Li
et al., 2013) reveals alterations of directional connec-
tivity in patients with Alzheimer disease, pointing to
the importance of this kind of knowledge in clinical
analysis. (Smith et al., 2011) also notes this, and
separates methods capable of describing directional
connectivity in three categories: lag-based methods
(Rodrigues and Andrade, 2014, See review in), such
as Granger causality (Granger, 1969) ; conditional
independence methods, such as Bayesian networks
(BN)(Mumford and Ramsey, 2014) and Dynamic
Causal Modeling (DCM)(Friston et al., 2003), and
higher-order statistics, such as Patel’s pairwise
conditional probability approach (Patel et al., 2006).
Dynamic Bayesian Networks (DBNs) can be re-
garded as a directed functional connectivity approach
(and thus, are used for exploratory analyses, instead
of confirmatory, such as DCM) that overcomes the
limitations of acyclicity imposed by the traditional
Bayesian network framework. They are Bayesian net-
works with nodes representing the processes at dif-
ferent time points, given a Markovianity assumption;
thus, cycles may be present as a result of temporal dy-
namics. Besides, the Bayesian network framework is
able to differentiate direct from indirect connections
(or, marginal dependences) between nodes by means
of conditional probabilities (Valdes-Sosa et al., 2011).
DBNs were applied to fMRI data in many studies (Ide
et al., 2014; Bielza and Larra
˜
naga, 2014) and were
modeled either with discrete data (with the condi-
tional probability distributions (CPD) are represented
by a probability table) (Rajapakse and Zhou, 2007;
Burge et al., 2009) or with continuous data through
a gaussianity assumption (Zhang et al., 2005; Chen
et al., 2012; Wu et al., 2014). The advantage of the
first case is that nonlinear processes are better mod-
eled, at the cost of reducing the original signal to dis-
crete values and requiring more samples, while in the
second case, processes are considered linear, but don’t
have to be discretized.
From the fact that the current state of knowledge
in hippocampal sub-fields connectivity and availabil-
ity of data demands a exploratory approach, this study
used DBNs as a tool for finding directed functional
connectivity between the hippocampal sub-fields of
the a resting state brain. This helps advance cur-
rent knowledge on the function of the medial tem-
poral lobe by introducing information on the direc-
tion of the connections, instead of only undirected and
pairwise correlations between regions. The model is
learned by an algorithm based on the search and score
paradigm, and uses the mutual information criterion
(MIT) (de Campos, 2006; Vinh et al., 2011a) to eval-
uate the goodness-of-fit of a DBN structure to the data
employed. Time-series data is obtained by extracting
average ROI time-series from the BOLD fMRI data
with coregistered hippocampal sub-field labels ob-
tained through an automatic segmentation algorithm
applied to structural T1 and T2-weighted structural
images. The highest scoring DBN structure found
for one subject shows the use of DBNs as a promis-
ing way to map the directed functional connectivity of
medial temporal lobe sub-structures using ultra-high
resolution neuroimaging.
2 METHODS
2.1 MRI Data
Images were collected from older adults diagnosed
with late-life depression, at the Department of Radi-
ology of University of Pittsburgh (NIH Project num-
ber 1R01MH111265-01). Scanning was done on a
7T human MRI system (Magnetom, Siemens Med-
ical Systems, Erlangen, Germany) with a 20-to-8-
channel transmit and 32-channel adjustable receive
coil (Ibrahim et al., 2013).
Structural T1 and T2-weighted images were used
for identifying the subregions with the automatic
segmentation. An axial, whole-brain T1 was ob-
tained with a Magnetization Prepared Rapid Gra-
dient Echo (MPRAGE) sequence with 3D orien-
tation, isotropic resolution 0.7mm3 and parame-
ters TR/TI/TE=3000/1200/2.47 ms, 6 flip angle,
FoV=192 × 220, 280 × 320 acquisition matrix and
an acquisition time 10 min and 31 s. Axial, whole-
brain T2-weighted images used a 3D Turbo Spin Echo
(TSE) sequence with 0.37 × 0.37mm in plane reso-
Dynamic Bayesian Network Modeling of Hippocampal Subfields Connectivity with 7T fMRI: A Case Study
179

lution and 1.5mm slice thickness; parameters were
TR/TE=7900/56 ms, 130 flip angle, FoV=165 × 189,
32 interleaved slices and acquisition time 5 min
and 25 s. Finally, 4D axial, whole-brain (exclud-
ing the cerebellum) BOLD fMRI data were acquired
with a gradient echo planar (EPI) sequence with 155
time points and 2.3mm isotropic voxel size, 46 in-
terleaved slices, TR/TE=2500/20 ms, 70 flip angle,
FoV=1546 × 1546, 96 × 96 acquistion matrix and ac-
quisition time 6 min and 27 s. Patients rested with
eyes open and lights off.
2.2 Segmentation and Preprocessing
Three ROIs are considered in the model: the den-
tate gyrus (DG), the four fields of the cornu ammo-
nis (CA14) (considered as one) and the entorhinal
cortex (ERC) from only one hemisphere of a subject
(Bartsch, 2012, See). The reason for choosing only
these is that as the number of nodes included in a
DBN model grows, the number of time points needed
for learning need to grow as well (Koller and Fried-
man, 2009) — and, with one subject, only 155 time
points are available. The ROIs are identified in the
T2-weighted images using an automatic segmentation
procedure applied to the images using the ASHS soft-
ware developed by (Yushkevich et al., 2015). ASHS
uses a multi-atlas label fusion approach for obtaining
the hippocampal subfields with T1 and T2-weighted
images (For a review of methods, see Dill et al.,
2015). A 7T atlas, based on images obtained in the
PREDICT-MR study (Wisse et al., 2014), tested with
ASHS in (Wisse et al., 2016) and made available pub-
licly, was used.
Preprocessing and image registering was done
with the statistical parametric mapping software
(SPM12) (Penny et al., 2011). Functional images
were slice-time corrected (reference slice was the
temporally middle slice with sinc interpolation), mo-
tion corrected (mean scan was used as reference with
mutual information as the similarity metric and 4th
degree B-spline interpolation), and smoothed with a
Gaussian smoothing kernel (FWHM of 8mm). The
T2-weighted images and segmentation labels are reg-
istered and resliced to match the functional image.
Time-series from the voxels of the ROIs are extracted
from the functional image according the aligned seg-
mentation labels. A mean intensity normalization is
applied: the average value of the ROI in each time
point is subtracted from each time-series, and normal-
ization is applied, with values ranging from -1 to 1.
Time-series of each ROIs are then averaged, yielding
one time-series for each ROI (Ide et al., 2014).
Time-series extracted from the ROIs are usually
non-stationary and present DC level trends that hin-
der the ability to use sequential time points for train-
ing a DBN (i.e., the supposition of ergodicity). These
trends are usually considered irrelevant to the model
and thus are removed by linear detrending methods
(Ide et al., 2014) and normalization. However, one
must consider the possibility that these trends can be
non-linear and present discontinuities, so that tradi-
tional detrending methods may not achieve stationary
time-series. Therefore, the Singular Spectrum Analy-
sis (SSA) technique is an interesting option due to its
model-free characteristic and ability to remove com-
plex trends (i.e., not just linear or quadratic) and dis-
continuities with very few specification parameters
(Golyandina and Zhigljavsky, 2013). We employ this
technique in the present work, by applying to each
time-series obtained from the ROIs. SSA’s main idea
is to ”decompose the original series into the sum of a
small number of independent and interpretable com-
ponents such as a slowly varying trend, oscillatory
components and structure-less noise” (Hassani, 2007)
and reconstruct it according to the components de-
sired — in case, the lowest oscillatory components
(which can be regarded as containing the direct cur-
rent (DC) level), represented by the largest eigen-
values obtained in the Singular Value Decomposition
(SVD) of the time-series’ embedding matrix (Golyan-
dina and Zhigljavsky, 2013).
2.3 Learning Discrete Dynamic
Bayesian Networks
We start by defining a Dynamic Bayesian Network
(DBN). Let X
(t)
= {X
(t)
1
, X
(t)
2
, . . . , X
(t)
N
}be a set of
N time-series of length T , where X
(t)
i
is a random
variable representing the process X
i
at time slice
t. A DBN models the joint probability distribution
X
(1)
∪ X
(2)
. . . ∪ X
(T )
by means of a directed acyclic
graph (DAG) G encoding the conditional indepen-
dences between the random variables of the sys-
tem (Friedman et al., 1998). Three assumptions are
made: (1) first order Markovianity, i.e., ∀t, (X
(t+1)
⊥
X
(0:t−1)
|X
(t)
), (2) stationarity, i.e., P(X
(t)
|X
(t−1)
) is
independent of t, and (3) Markov causal condition,
i.e., (X
(t)
i
⊥ Nd(X
(t)
i
)|Pa(X
(t)
i
)), where Nd(X
(t)
i
) are
the non-descendant nodes of X
(t)
i
, and Pa(X
(t)
i
) are
the parent nodes of X
(t)
i
in the DAG G (Neapolitan
et al., 2004). Thus, this permits the decomposition of
the joint probability distribution of all the processes
of the system as:
P(X
(0:t)
) = P(X
(0)
)
T
∏
t=1
P(X
(t)
|X
(t−1)
) (1)
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
180

X
(t−1)
X
(t)
Y
(t−1)
Y
(t)
Z
(t−1)
Z
(t)
A
(t−1)
A
(t)
B
(t−1)
B
(t)
C
(t−1)
C
(t)
A
B
C
(a) (b) (c)
Figure 1: Examples of DBNs. (a) presents a DBN with only
inter time-slice edges, which can be represented as a cycli-
cal graph as shown in (b) (used in the present study). (c)
presents a DBN with both intra and inter time-slice edges.
and the further decomposition of the joint transi-
tion probability P(X
(t)
|X
(t−1)
) as:
P(X
(t)
|X
(t−1)
) =
N
∏
i=1
P(X
(t)
i
|Pa(X
(t)
i
) (2)
These decompositions thus permit both network
inference algorithms for unknown variables and the
formulation of scoring metrics and other structure
learning procedures from data (such as the one used
and presented further) (Neapolitan et al., 2004).
DBNs can include edges between nodes in a same
time slice t (intra time slice) and between time slices
t − 1 and t (inter time slice) (time slices t − 2 and
on can be included by relaxing the Markovianity as-
sumption to permit higher orders, such as in (Xuan
et al., 2012) and (Santos and Maciel, 2014)). Figure 1
(c) illustrates this type of network. However, for sim-
plicity, many authors consider DBNs including only
inter time slice edges (Xuan et al., 2012; Smith et al.,
2006). This last case permits an equivalent graph rep-
resentation without specifying time-series at different
time points and thus without the acyclicity restriction,
such as shown in Figure 1(a,b).
DBN graphs can be learned from data through
many approaches, among which are conditional in-
dependence tests, score metrics, and model averag-
ing (such as Markov Chain Monte Carlo) (Koller
and Friedman, 2009). In this paper we employ the
score metrics approach for discrete models (dDBNs),
in which, after searching through a space of possi-
ble graph structures, a scoring function determines
how good this structure is given the data. Partic-
ularly, we employ the GlobalMIT method, imple-
mented in a Matlab/C++ toolkit (Vinh et al., 2011a).
GlobalMIT adapts the MIT scoring metric proposed
by (de Campos, 2006), devised initially for static
Bayesian networks, to DBNs and uses an optimized
search algorithm operating under polynomial time
(Vinh et al., 2011b). The MIT scoring metric eval-
uates the goodness-of-fit of a network ”as the total
mutual information shared between each node and its
parents, penalized by a term which quantifies the de-
gree of statistical significance of this shared informa-
tion” (Xuan et al., 2012), and was shown to compete
favorably with other scoring metrics such as Bayesian
Dirichlet equivalence (BDe) and Bayesian Informa-
tion Critertion (BIC) (de Campos, 2006). For a can-
didate graph G with N nodes and their corresponding
{r
1
, . . . , r
n
} discrete states, and a dataset D with N
D
observations, the MIT score is defined as (de Cam-
pos, 2006):
S
MIT
(G : D) =
=
N
∑
i=1
Pa(X
i
)6=
/
0
{2N
D
·I(X
i
, Pa(X
i
)) −
s
i
∑
j=1
χ
α,l
iσ( j)
}
(3)
where I(X
i
, Pa(X
i
)) is the mutual information be-
tween X
i
and its parents, and χ
α,l
iσ( j)
is the value such
that p(χ
2
(l
i
j) ≤ χ
α,l
i j
) = α (the chi-square distribu-
tion at significance level 1 − α, with the term l
iσ
i
( j)
defined as:
l
iσ
i
( j)
=
=
(
(r
i
− 1)(r
iσ
i
( j)
− 1)
∏
j−1
k=1
r
iσ
i
(k)
, j = 2, . . . , s
i
(r
i
− 1)(r
iσ
i
( j)
− 1), j = 1
(4)
where σ
i
= {σ
i
(1), . . . , σ
i
(s
i
)} is any permuta-
tion of the index set {1, . . . , s
i
} of Pa(X
i
), with the
first variable having the greatest number of states, the
second having the second, and so on.
The GlobalMIT algorithm then investigates, for
each node, every potential parent set of increasing car-
dinality until the globally optimal solution is found.
This guarantees a polynomial worst-case time com-
plexity, given the assumptions that the DBN includes
only inter time slices (thus eliminating the necessity
of checking acyclicity of candidate graphs) and vari-
ables have the same number of discrete states (con-
sidering also the MIT scoring criterion is used in the
algorithm) (Vinh et al., 2011a). GlobalMIT is also
deterministic, and thus have advantage over stochas-
tic global optimization methods, since it does not get
stuck in local minima (Vinh et al., 2011b). The algo-
rithm also uses the following decomposition property
of mutual information:
I(X
i
, Pa(X
i
) ∪ X
j
) = I(X
i
, Pa(X
i
)) + I(X
i
, X
j
|Pa(X
i
))
(5)
which permits an incremental calculation of mutual
information, with cached intermediary values to pre-
vent redundant computation, and thus a better effi-
ciency.
Dynamic Bayesian Network Modeling of Hippocampal Subfields Connectivity with 7T fMRI: A Case Study
181
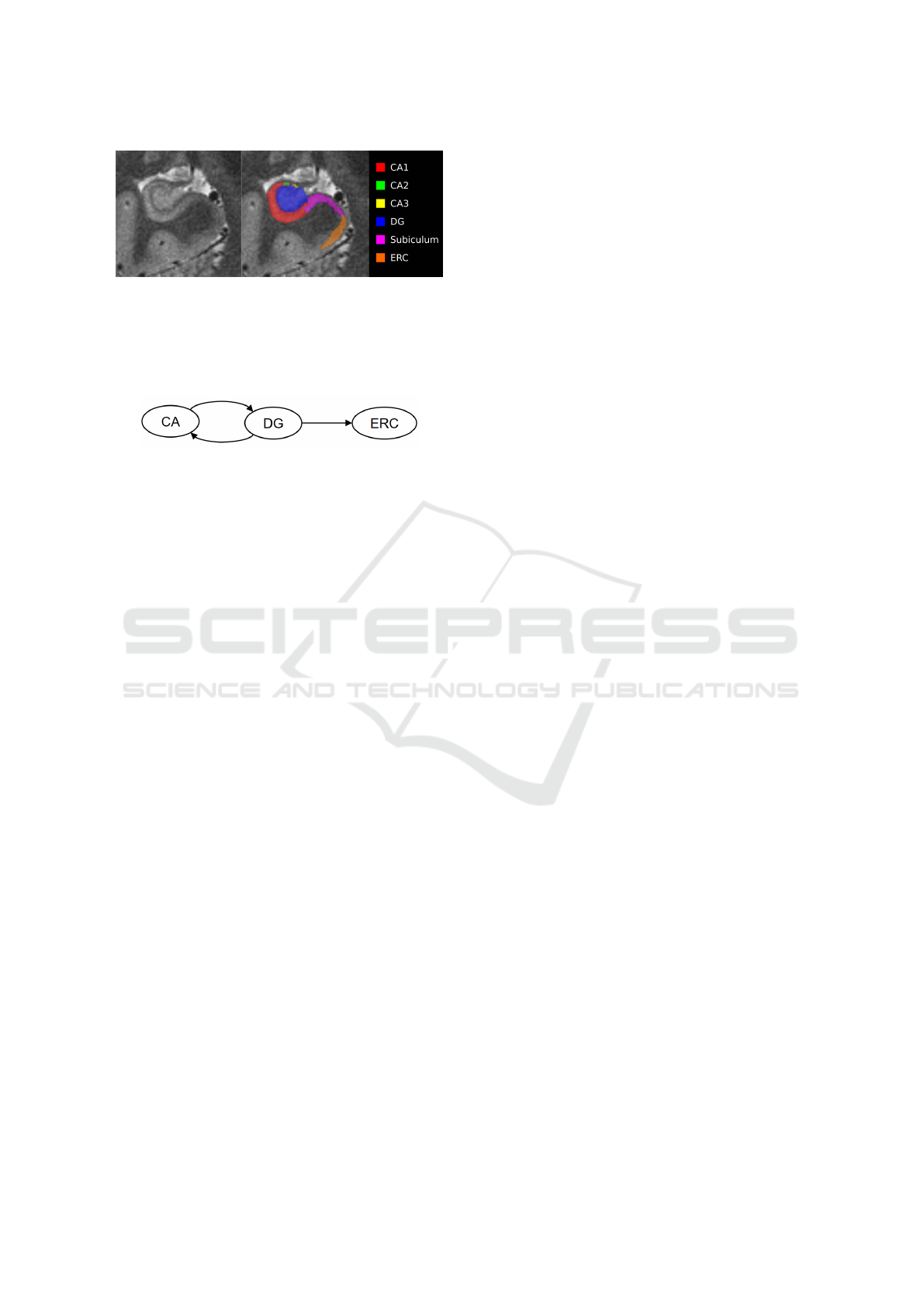
Figure 2: Resulting labels from the automatic segmenta-
tion procedure implemented in ASHS (Yushkevich et al.,
2015). Labels are shown at the T2-weighted structural im-
age with 0.x0.x0. mm, but are afterwards registered to the
functional images for the ROI time-series extraction proce-
dure and network learning.
Figure 3: Highest scoring DBN structure with the Glob-
alMIT score applied to the hippocampal subfields nodes.
CA stands for cornu ammonis; DG, dentate gyrus and ERC,
entorhinal cortex.
3 RESULTS AND DISCUSSION
Automatic segmentation of hippocampal sub-fields
was applied to T1 and T2-weighted images of one
subject, as shown in Figure 2. After the registra-
tions and preprocessing, three ROI time-series were
extracted and from the fMRI image: CA, DG and
ERC. Then, SSA technique was applied to remove
complex trends in the signals - time-series were de-
composed into 50 components, and 2 of the lowest
oscillatory components (related to the largest eigen-
values of the SVD decomposition) were removed. Fi-
nally, data were discretized to 5 bins (d = 5) and the
GlobalMIT algorithm was applied for obtaining the
discrete DBN structure. After structure search, the
high scoring graph was the one presented in Figure 3,
with significance level of α = 0.95.
The network structure found reveals a mutual con-
nection between CA and DG fields and a directed
connection from DG to ERC. This indicates that the
connection between CA and ERC is mediated by the
DG, and this finding may explain why the DG is more
functionally connected to the parahippocampal and
cingulate cortices than the CA (de Flores et al., 2015)
((Libby et al., 2012) shows that connections between
the hippocampal subfields and the parahippocampal
cortex are mediated by the ERC). A fuller understand-
ing of these connections, however, would require an
analysis at group level, since this reflects results found
for one subject.
4 CONCLUSION & FUTURE
WORK
Advances with ultra-field MRI, yielding high-
resolution fMRI data, and automatic segmentation
procedures for sub-field identification are opening the
way for studies on sub-field level connectivity analy-
ses in the brain. In this study, we demonstrated the
feasibility of a directed functional connectivity analy-
sis of hippocampal sub-fields data obtained with ultra-
high resolution neuroimaging, automatic segmenta-
tion and DBN models. The resulting DBN models are
able to identify direction of connections and differen-
tiate direct from indirect connections between ROIs,
which may better explain the function of each hip-
pocampal sub-field. Also, in the preprocessing steps,
we included a novel application of the SSA technique
for removing complex trends and discontinuities from
time-series in a flexible, model-free way and with
very few specification parameters.
Results, however, were obtained for only one sub-
ject, and thus the next step of work will be a group
level analysis, including data from multiple subjects,
for a more complete interpretation of results. DBN
group analysis techniques are described in (Li et al.,
2007) and (Li et al., 2008), in which three main
groups are distinguished: ”the virtual-typical- subject
(VTS) approach which pools or averages group data
as if they are sampled from a single, hypothetical vir-
tual typical subject; the individual-structure (IS) ap-
proach that learns a separate BN for each subject, and
then finds commonality across the individual struc-
tures, and the common-structure (CS) approach that
imposes the same network structure on the BN of ev-
ery subject, but allows the parameters to differ across
subjects” (Li et al., 2008). An investigation of inter-
subject conformities and variations can yield impor-
tant results for functional and clinical studies(Bielza
and Larra
˜
naga, 2014) on the hippocampus and medial
temporal lobe, such as identification of early stages of
progression of Alzheimer’s disease (Das et al., 2013).
Another future step consists in including more
ROIs, for a more complete network model — until
now, usage of data of only one subject (thus, with
only 155 time points) limits the number of nodes to
be included in the network. Important regions to be
added are the remaining parts of the medial temporal
lobes — the subiculum (part of the hippocampus), the
perirhinal cortex (PRC) and the parahippocampal cor-
tex (PHC)— and also the anterior and posterior cin-
gulate cortices; all of these from both the right and left
hemispheres, as investigated in previous work such as
(Lacy and Stark, 2012) and (de Flores et al., 2015).
A study with a seed-to-voxel correlation analysis will
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
182

be also conducted for identifying relevant activation
areas to be included (such as described in (Ide et al.,
2014)).
Requirements of data for learning DBNs can be
naturally overcome with some types of analysis at
group level — (Burge et al., 2009), for example,
employs a cross-validation procedure, including data
from multiple subjects. The requirements can also
be overcome by means of multi-modal learning tech-
niques, which use data from multiple types of im-
ages to reduce the search space of networks or mod-
els. (Schulz et al., 2004), for example, performs
an integrated MEG and fMRI for connectivity anal-
ysis, (Rykhlevskaia et al., 2008) performs a survey of
methods integrating structural and functional images
for connectivity analysis, and (Zhu et al., 2014) sur-
veys of methods integrating diffusion tensor imaging
(DTI) with functional images. (Iyer et al., 2013), for
example, uses DTI data as a baseline matrix for an
Bayesian network structure learning procedure — an
important insight that may be further incorporated.
ACKNOWLEDGEMENTS
This work was supported by grants T32 MH019986
and P30 MH090333 from the National Institutes
of Health (USA), grant 2016/02621-0 from So
Paulo Research Foundation (FAPESP/Brazil), grant
475064/2013-5 from National Council of Techno-
logical and Scientific Development (CNPq/Brazil),
and grant BEX 13385/13-5 from Capes Foundation
(Brazil).
REFERENCES
Bartsch, T. (2012). The clinical neurobiology of the hip-
pocampus: An integrative view, volume 151. Oxford
University Press.
Bielza, C. and Larra
˜
naga, P. (2014). Bayesian networks in
neuroscience: a survey. Frontiers in computational
neuroscience, 8:131.
Burge, J., Lane, T., Link, H., Qiu, S., and Clark, V. P.
(2009). Discrete dynamic bayesian network analysis
of fmri data. Human brain mapping, 30(1):122–137.
Chen, R., Resnick, S. M., Davatzikos, C., and Herskovits,
E. H. (2012). Dynamic bayesian network model-
ing for longitudinal brain morphometry. NeuroImage,
59(3):2330 – 2338.
Das, S. R., Pluta, J., Mancuso, L., Kliot, D., Orozco, S.,
Dickerson, B. C., Yushkevich, P. A., and Wolk, D. A.
(2013). Increased functional connectivity within me-
dial temporal lobe in mild cognitive impairment. Hip-
pocampus, 23(1):1–6.
de Campos, L. M. (2006). A scoring function for learning
bayesian networks based on mutual information and
conditional independence tests. J. Mach. Learn. Res.,
7:2149–2187.
de Flores, R., Mutlu, J., Bejanin, A., Tomadesso, C., Lan-
deau, B., M
´
ezenge, F., de La Sayette, V., Eustache,
F., and Ch
´
etelat, G. (2015). Intrinsic connectivity of
hippocampal subfields in normal elderly and its distur-
bance in mci patients. Alzheimer’s & Dementia: The
Journal of the Alzheimer’s Association, 11(7):P61.
Duyn, J. H. (2012). The future of ultra-high field {MRI}
and fmri for study of the human brain. NeuroIm-
age, 62(2):1241 – 1248. 20 {YEARS} {OF} fMRI20
{YEARS} {OF} fMRI.
Friedman, N., Murphy, K., and Russell, S. (1998). Learn-
ing the structure of dynamic probabilistic networks. In
Proceedings of the Fourteenth conference on Uncer-
tainty in artificial intelligence, pages 139–147. Mor-
gan Kaufmann Publishers Inc.
Friston, K. J. (2011). Functional and effective connectivity:
a review. Brain connectivity, 1(1):13–36.
Friston, K. J., Harrison, L., and Penny, W. (2003). Dynamic
causal modelling. Neuroimage, 19(4):1273–1302.
Golyandina, N. and Zhigljavsky, A. (2013). Singular Spec-
trum Analysis for Time Series. SpringerBriefs in
Statistics. Springer.
Granger, C. W. (1969). Investigating causal relations
by econometric models and cross-spectral methods.
Econometrica: Journal of the Econometric Society,
pages 424–438.
Hassani, H. (2007). Singular spectrum analysis: method-
ology and comparison. Journal of Data Science,
5(2):239–257.
Ibrahim, T. S., Zhao, Y., Krishnamurthy, N., Raval, S. B.,
Zhao, T., Wood, S., and Kim, J. (2013). 20-to-8 chan-
nel tx array with 32-channel adjustable receive-only
insert for 7t head imaging. In The 21th ISMRM An-
nual Meeting, page 4408.
Ide, J. S., Zhang, S., and Chiang-shan, R. L. (2014).
Bayesian network models in brain functional connec-
tivity analysis. International Journal of Approximate
Reasoning, 55(1):23–35.
Iyer, S. P., Shafran, I., Grayson, D., Gates, K., Nigg, J. T.,
and Fair, D. A. (2013). Inferring functional connec-
tivity in mri using bayesian network structure learning
with a modified pc algorithm. Neuroimage, 75:165–
175.
Jeneson, A. and Squire, L. R. (2012). Working memory,
long-term memory, and medial temporal lobe func-
tion. Learning & Memory, 19(1):15–25.
Koller, D. and Friedman, N. (2009). Probabilistic graphical
models: principles and techniques. MIT press.
Lacy, J. W. and Stark, C. E. (2012). Intrinsic functional
connectivity of the human medial temporal lobe sug-
gests a distinction between adjacent mtl cortices and
hippocampus. Hippocampus, 22(12):2290–2302.
Li, J., Wang, Z. J., and McKeown, M. J. (2007). A frame-
work for group analysis of fmri data using dynamic
bayesian networks. In 2007 29th Annual International
Conference of the IEEE Engineering in Medicine and
Biology Society, pages 5991–5994. IEEE.
Dynamic Bayesian Network Modeling of Hippocampal Subfields Connectivity with 7T fMRI: A Case Study
183

Li, J., Wang, Z. J., Palmer, S. J., and McKeown, M. J.
(2008). Dynamic bayesian network modeling of fmri:
a comparison of group-analysis methods. Neuroim-
age, 41(2):398–407.
Li, R., Wu, X., Chen, K., Fleisher, A., Reiman, E., and
Yao, L. (2013). Alterations of directional connectiv-
ity among resting-state networks in alzheimer disease.
American Journal of Neuroradiology, 34(2):340–345.
Libby, L. A., Ekstrom, A. D., Ragland, J. D., and
Ranganath, C. (2012). Differential connectivity
of perirhinal and parahippocampal cortices within
human hippocampal subregions revealed by high-
resolution functional imaging. The Journal of Neu-
roscience, 32(19):6550–6560.
Maruszak, A. and Thuret, S. (2015). Why looking at the
whole hippocampus is not enougha critical role for
anteroposterior axis, subfield and activation analyses
to enhance predictive value of hippocampal changes
for alzheimers disease diagnosis. 2015: Which new
directions for Alzheimer’s disease?, page 6.
Mumford, J. A. and Ramsey, J. D. (2014). Bayesian net-
works for fmri: a primer. Neuroimage, 86:573–582.
Neapolitan, R. E. et al. (2004). Learning bayesian networks.
Patel, R. S., Bowman, F. D., and Rilling, J. K. (2006). A
bayesian approach to determining connectivity of the
human brain. Human brain mapping, 27(3):267–276.
Penny, W. D., Friston, K. J., Ashburner, J. T., Kiebel, S. J.,
and Nichols, T. E. (2011). Statistical parametric map-
ping: the analysis of functional brain images. Aca-
demic press.
Rajapakse, J. C. and Zhou, J. (2007). Learning effective
brain connectivity with dynamic bayesian networks.
Neuroimage, 37(3):749–760.
Rykhlevskaia, E., Gratton, G., and Fabiani, M. (2008).
Combining structural and functional neuroimaging
data for studying brain connectivity: a review. Psy-
chophysiology, 45(2):173–187.
Santos, F. P. and Maciel, C. D. (2014). A pso approach for
learning transition structures of higher-order dynamic
bayesian networks. In Biosignals and Biorobotics
Conference (2014): Biosignals and Robotics for Bet-
ter and Safer Living (BRC), 5th ISSNIP-IEEE, pages
1–6.
Schulz, M., Chau, W., Graham, S. J., McIntosh, A. R., Ross,
B., Ishii, R., and Pantev, C. (2004). An integrative
meg–fmri study of the primary somatosensory cortex
using cross-modal correspondence analysis. NeuroIm-
age, 22(1):120–133.
Smith, S. M., Miller, K. L., Salimi-Khorshidi, G., Web-
ster, M., Beckmann, C. F., Nichols, T. E., Ramsey,
J. D., and Woolrich, M. W. (2011). Network mod-
elling methods for fmri. Neuroimage, 54(2):875–891.
Smith, V. A., Yu, J., Smulders, T. V., Hartemink, A. J., and
Jarvis, E. D. (2006). Computational inference of neu-
ral information flow networks. PLoS computational
biology, 2(11):e161.
Valdes-Sosa, P. A., Roebroeck, A., Daunizeau, J., and Fris-
ton, K. (2011). Effective connectivity: influence,
causality and biophysical modeling. Neuroimage,
58(2):339–361.
Vinh, N. X., Chetty, M., Coppel, R., and Wangikar, P. P.
(2011a). Globalmit: learning globally optimal dy-
namic bayesian network with the mutual information
test criterion. Bioinformatics, 27(19):2765–2766.
Vinh, N. X., Chetty, M., Coppel, R., and Wangikar, P. P.
(2011b). Polynomial time algorithm for learning glob-
ally optimal dynamic bayesian network. In Interna-
tional Conference on Neural Information Processing,
pages 719–729. Springer.
Wang, C., Xu, J., Zhao, S., and Lou, W. (2015). Graph the-
oretical analysis of eeg effective connectivity in vas-
cular dementia patients during a visual oddball task.
Clinical Neurophysiology.
Wisse, L., Kuijf, H., Honingh, A., Wang, H., Pluta, J.,
Das, S., Wolk, D., Zwanenburg, J., Yushkevich, P.,
and Geerlings, M. (2016). Automated hippocampal
subfield segmentation at 7t mri. American Journal of
Neuroradiology.
Wisse, L. E., Biessels, G. J., Heringa, S. M., Kuijf, H. J.,
Luijten, P. R., Geerlings, M. I., Group, U. V. C. I.
V. S., et al. (2014). Hippocampal subfield volumes
at 7t in early alzheimer’s disease and normal aging.
Neurobiology of aging, 35(9):2039–2045.
Wu, X., Wen, X., Li, J., and Yao, L. (2014). A new dynamic
bayesian network approach for determining effective
connectivity from fmri data. Neural Computing and
Applications, 24(1):91–97.
Xuan, N., Chetty, M., Coppel, R., and Wangikar, P. P.
(2012). Gene regulatory network modeling via global
optimization of high-order dynamic bayesian net-
work. BMC bioinformatics, 13(1):131.
Yushkevich, P. A., Pluta, J. B., Wang, H., Xie, L., Ding, S.-
L., Gertje, E. C., Mancuso, L., Kliot, D., Das, S. R.,
and Wolk, D. A. (2015). Automated volumetry and
regional thickness analysis of hippocampal subfields
and medial temporal cortical structures in mild cogni-
tive impairment. Human brain mapping, 36(1):258–
287.
Zhang, L., Samaras, D., Alia-Klein, N., Volkow, N., and
Goldstein, R. (2005). Modeling neuronal interactiv-
ity using dynamic bayesian networks. In Advances in
neural information processing systems, pages 1593–
1600.
Zhu, D., Zhang, T., Jiang, X., Hu, X., Chen, H., Yang, N.,
Lv, J., Han, J., Guo, L., and Liu, T. (2014). Fusing dti
and fmri data: a survey of methods and applications.
Neuroimage, 102:184–191.
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
184
