
Proposal of New Tracer Concentration Model in Lung PCT Study
Comparison with Commonly Used Gamma-variate Model
Maciej Browarczyk
1
, Renata Kalicka
1
and Seweryn Lipiński
2
1
Department of Biomedical Engineering, Gdańsk University of Technology, Narutowicza 11/12 Street, Gdańsk, Poland
2
Department of Electrical Engineering, Power Engineering, Electronics and Automation,
University of Warmia and Mazury, Olsztyn, Poland
Keywords: Modelling, pCT, Gamma-Variate, Gauss, Rayleigh.
Abstract: Perfusion computed tomography (pCT) is one of the methods that enable non-invasive imaging of the
hemodynamics of organs and tissues. On the basis of pCT measurements, perfusion parameters such as
blood flow (BF), blood volume (BV), mean transit time (MTT) and permeability surface (PS) are calculated
and then used for quantitative evaluation of the tissue condition. To calculate perfusion parameters it is
necessary to approximate concentration-time curves using regression function. In this paper we compared
three regression functions: first commonly used gamma-variate function, second and third Gauss and
Rayleigh functions, not previously used for this purpose. The Gauss function showed clear advantage over
the others when considering results of simulated data analysis. Actual measurements analysis confirmed
conclusions from simulated data analysis. It was showed that contrary to widely accepted belief, the
differences between rising and falling edge slope angles of concentration-time curves are inconsiderable.
For that reason, it can be assumed that rising and falling edges are symmetrical. The main conclusion is that
the Gauss function gives a more robust fit than the widely used gamma-variate function when modelling
concentration-time curves in lung pCT studies.
1 INTRODUCTION
Perfusion computed tomography (pCT) is one of the
methods that enable non-invasive imaging of the
hemodynamics of organs and tissues. On the basis of
pCT measurements, perfusion parameters such as
blood flow (BF), blood volume (BV), mean transit
time (MTT) and permeability surface (PS) are
calculated and then used for quantitative evaluation
of the tissue condition. Usefulness of perfusion
parameters has been proved in the diagnosis of brain
(Wintermark et al., 2008), kidneys (Zhao et al.,
2010), liver (Mírka et al., 2010), pancreas
(Balthazar, 2011) and spleen (Sauter et al., 2012). In
the case of lungs, as in other organs, perfusion
imaging is particularly useful for diagnosing cancer
(Cao, 2011). The method allows not only for
establishing the tumour size and location (Nakano et
al., 2013), but may also provide important predictive
information concerning tumour vasculature (Ng and
Goh, 2010). Lung pCT measurements can also help
in the diagnosis of diabetic pulmonary
microangiopathy (Browarczyk et al., 2015; Kalicka
et al., 2015).
The pCT chest technique uses the intravenous
injection of a non-iodinated contrast agent (tracer)
and the sequential scanning of the chest when the
agent passes through the lungs for the first time
("first-pass"). The tissue concentration-time curve
c(t) is obtained for every pixel of the diagnosed
cross-section. The relationship between the arterial
input function tracer concentration c
AIF
(t) on
entering the region of interest (ROI) and the c(t)
measured within the ROI has been formulated on the
basis of the tracer kinetics theory.
To calculate perfusion parameters it is necessary
to approximate the data in the form of c(t) and c
AIF
(t)
measurements with regression function. The most
commonly used functions for this purpose are the
gamma-variate (Blomley and Dawson, 1997;
Jackson, 2004) and two- or three-exponential
functions (Kalicka and Pietrenko-Dąbrowska, 2007;
Srikanchana et al., 2004). However, regression
functions that have good properties when applied to
dynamic brain research (Kalicka and Pietrenko-
Dąbrowska, 2007) or carotid artery (Lampaskis et
al., 2009), demonstrate worse performance, for
instance, in the case of renal studies (Balvay et al.,
134
Browarczyk M., Kalicka R. and LipiÅ
ˇ
Dski S.
Proposal of New Tracer Concentration Model in Lung PCT Study - Comparison with Commonly Used Gamma-variate Model.
DOI: 10.5220/0006115101340140
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 134-140
ISBN: 978-989-758-212-7
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2008) or liver (Lampaskis et al., 2009). This
observation inspired us to compare the gamma-
variate function with the Gauss and the Rayleigh
functions in pCT lung studies. The Gauss and the
Rayleigh functions were not previously used for this
purpose. All of the functions were compared using
both actual and simulated pCT data.
2 MATERIAL AND METHODS
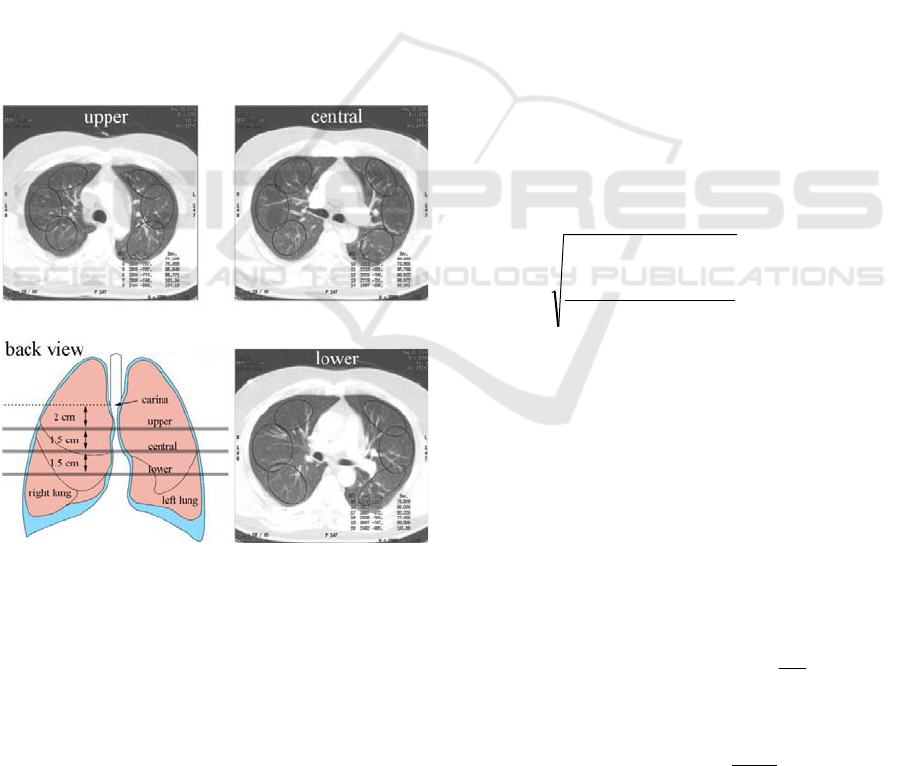
The clinical measurements were performed on a 64-
row Light Speed VCT CT scanner produced by GE
Healthcare USA. Pulmonary perfusion was axially
evaluated in three cross-sections: the upper, the
middle and the lower parts of the lungs (2 cm, 3.5
cm and 5 cm below carina, respectively, see figure
1), 12 s after the intravenous administration of 40 ml
a non-iodinated contrast medium at a rate of 4 ml/s.
Each of the three sequences consists of 89 scans,
with a resolution of 512 at 512 pixels, collected with
a sampling interval of 1 s.
Figure 1: Three cross-sections obtained from healthy
subject.
Two data sets were collected. The first data set
consists of actual measurements and the second data
set was simulated. Simulated data were used for
extended analysis of error propagation for all
considered regression functions. Each data set, both
measured and simulated, consists of three
concentration-time curves, one per lung region:
arterial input function (AIF), blood vessels and
parenchyma. The first data set was created from the
actual measurements obtained from 5 healthy
subjects, 2 females and 3 males, aged 33-67. The
second data set was simulated using the actual
measurements obtained from another healthy subject
(female, 67 y/o, non-smoking with no diagnosed
acute or chronic diseases affecting pulmonary
functions). All patients received written information
about the study, then gave written consent to
participate. The study was approved by the
Independent Commission on Bioethics Committee
for Scientific Research at the Medical University of
Gdańsk.
The simulations were conducted in the following
way: 100 pixels were manually chosen from the
upper, the central and the lower cross-sections. The
selected pixels provide j=1,...,100 concentration-
time curves c
j
(t
i
). Each curve consists i=1,...,89
measurements. The most typical concentration-time
curve c
typ
(t
i
) was calculated as a mean vector,
defined as sum of vectors c
j
(t
i
) divided by number of
vectors. The typical curves were used to perform
100 simulation runs c
sim
(t
i
):
R,
typsim ii
tcGtc
(1)
G(c
typ
(t
i
),R) is the random numbers generator of the
normal distribution with mean parameter c
typ
(t
i
) and
standard deviation R equal to the residual variance
of actual measurements:
100,
1
1
2
typ
j
jj
tctc
j
iji
R
(2)
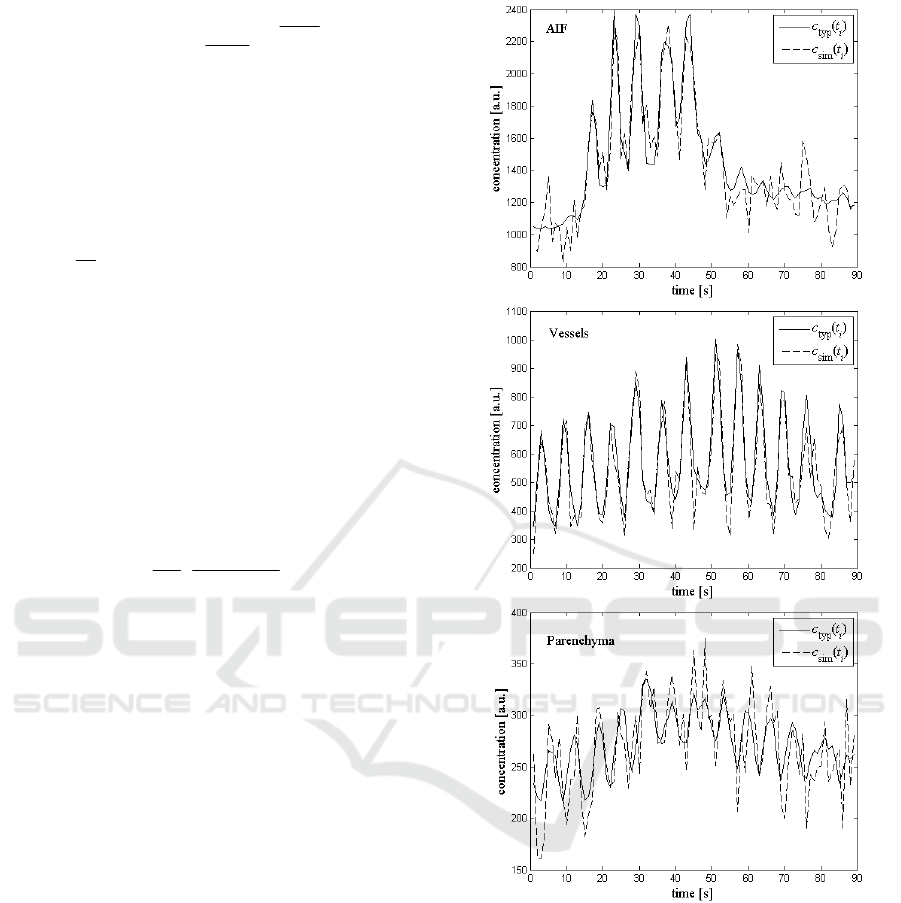
Figure 2 shows the c
typ
(t
i
) and an example of a
c
sim
(t
i
) for AIF, vessels and parenchyma.
The peaks and valleys (figure 2) are
characteristic for pCT lung results. They are caused
by the patient's breathing during the examination. In
further analysis we consider the peaks which
correspond to the phase of inspiration. The peaks
were detected using the function findpeaks
(Mathworks Matlab R2010a). Only the first passage
of tracer was modelled.
The following functions were chosen to be
compared:
gamma-variate function;
3
0
2
010321
,,,,
v
tt
v
ettvttvvvV
(3)
Gauss function;
3
2
2
1321
,,,
g
gt
egtgggG
(4)
Rayleigh;
Proposal of New Tracer Concentration Model in Lung PCT Study - Comparison with Commonly Used Gamma-variate Model
135
2
2
2
0
2
2
2
0
1021
,,,
r
tt
e
r
tt
rttrrR
(5)
where v
1
, v
2
, v
3
, g
1
, g
2
, g
3
, r
1
, r
2
are regression
function parameters; t
0
is arrival time of contrast
agent, determined empirically.
The values of model parameters v = [v
1
, v
2
, v
3
], g
= [g
1
, g
2
, g
3
] and r = [r
1
, r
2
] were calculated
according to the objective function:
OFppp
tctc
N
OF
p
n
N
i
ii
minarg,..,,
min,
1
21
1
0
2
mod
p
p
(6)
where N is number of time points, p is the parameter
vector equal to v, g and r for the gamma-variate, the
Gauss and the Rayleigh model functions c
mod
(t
i
,p),
respectively.
The BV parameter is the relative blood volume in
the considered ROI. It is defined as follows
(Calamante et al., 1999):
0
0
dttc
dttc
k
BV
AIF
H
(7)
where k
H
is the correction factor accounting for the
differences in the hematocrit of capillaries and large
vessels, ρ is the tissue density [g/cm3], and c(t) and
c
AIF
(t) are the concentration of contrast agent in ROI
and in AIF, respectively. In literature the values of
k
H
and ρ differ significantly (Chan and Siochi, 2011;
Cohen, 1966; Hopkins et al., 2007; Lilienfeld et al.,
1956; Pevsner et al., 2005; Praveenkumar et al.,
2011). In our research the precise values of k
H
and ρ
are not relevant. We assume k
H
/ρ = 1 for all the
considered types of tissue: AIF, vessels,
parenchyma.
Next, the models will be used to calculate the
blood volume BV defined by the equation 7, which
is the diagnostically important descriptor of lung
perfusion. Errors associated with measurements
propagate to the errors associated with model
parameters and in turn they propagate to errors of
perfusion parameter BV. The way of propagation
depends on the particular form of the regression
function. We will test different regression functions
to compare their built-in, inner potential to provide
accurate identification results. To get the aim we
will apply methodology and criteria of the error
propagation and of the sensitivity analysis.
There are two basic questions relating to the
Figure 2: Typical concentration-time curves c
typ
(t
i
) and an
example of the simulated curve c
sim
(t
i
) for AIF (upper),
vessels (middle) and parenchyma (lower).
identification of model parameters. Is the model
identifiable, i.e. whether there is a unique solution in
form of model parameters? Whether the parameters
can be designated on the basis of the measurements
with a satisfactory accuracy?
It is important to obtain unique estimates of all
model parameters. This problem is considered as
theoretical or a priori identifiability. Sometime a
model is theoretically identifiable, but process of
parameters estimation may produce such large errors
that occurs a loss of practical or a posteriori
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
136
identifiability.
Process of parameters estimation requires finding
minimum of objective function OF in the parameter
space. Dimension of the parameter space is equal to
the number of model parameters n
p
≤N, N is number
of measurements:
OFppp
tyty
N
OF
p
n
N
i
ii
minarg,..,,
min,
1
21
1
0
2
modmeas
p
p
(8)
where y
meas
(t
i
) is a set of measurements collected in
N time points t
i
and y
mod
(p,t
i
) is a model function
that depend on parameter vector p.
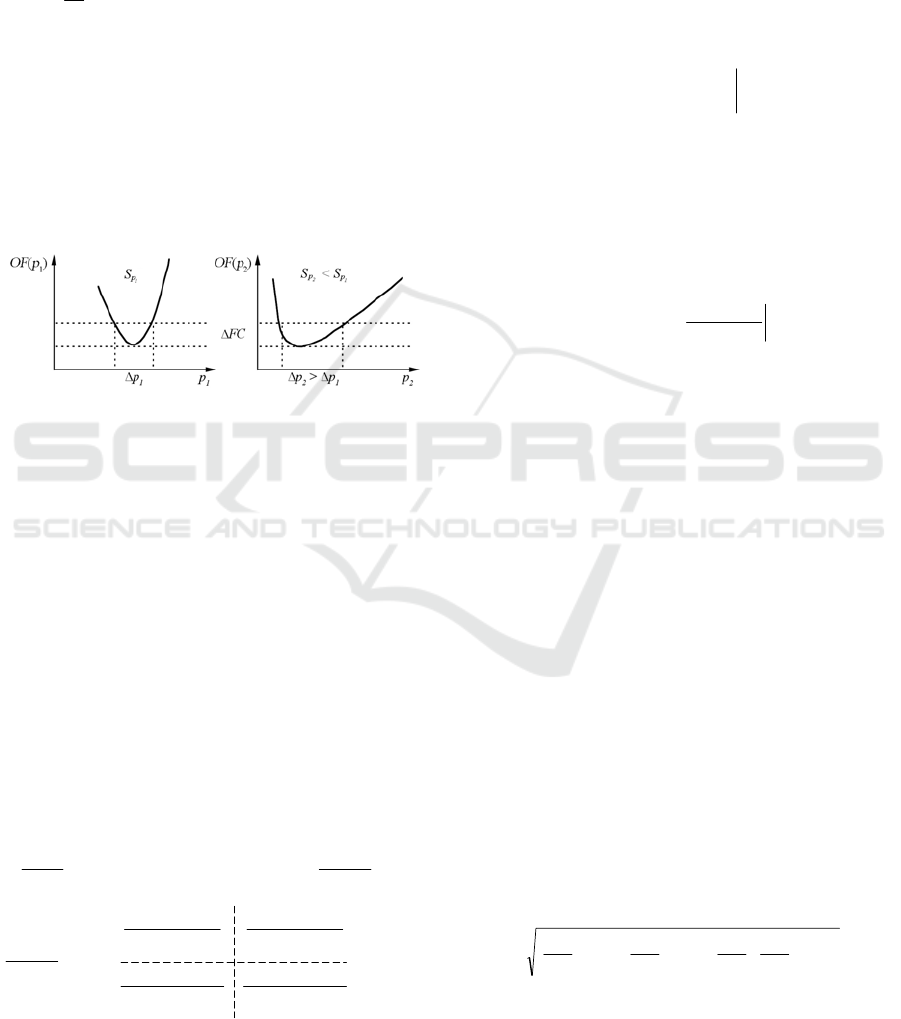
Let us analyse sensitivity S
p
of OF(p) with
respect to estimated parameters – for large and for
small sensitivity value. Assume that the resolution of
the OF measurement is OF, see Figure 3.
Figure 3: Dependence between the shape of OF and the
attainable accuracy for ∆p
1
and ∆p
2
> ∆p
1
for two model
parameters p
1
and p
2
and for the same measurement
resolution OF.
The larger sensitivity S
p
the smaller error p.
Therefore, for the same measurement resolution OF,
the attainable accuracy p differs depending on the
particular sensitivity function. The sensitivities are
dependent on the properties of the regression
function. Choice of the regression function of
desired properties is the aim of our investigation.
The relationship between sensitivity and error
one can present in an analytical form:
iiii
ttttOF ,,
modmeas
T
modmeas
pyypyy
(9)
where i = 1,...,N and p = [p
1
, p
2
,...,p
np
].
Differentiating OF with respect to the parameter
vector we obtain:
p
p
p
Nxn
n
NN
n
ii
p
t
p
t
p
t
p
t
tt
OF
),(),(
),(),(
,2
mod
1
mod
1mod
1
1mod
mod
mod
T
modmeas
pypy
pypy
S
p
y
p
y
pyy
p
(10)
S is the sensitivity matrix. Searching for error of
parameter estimates involves the searching for
variance-covariance matrix of the estimates. Under
simplifying assumptions (y
mod
(p,t
i
) is approximated
to a linear factor of Taylor series, the disturbances
are uncorrelated, the expected value of the
disturbances is zero, and covariance is constant and
equal to σ
2
) the variance-covariance matrix P in the
vicinity of the minimum of OF, takes the form of
(Cobelli et al., 2002; Enderle et al., 2000;
Semmelow, 2005):
minarg
1
T2
pp
SSPP
pp
OF
opt
xnn
opt
pp
(11)
The matrix P is symmetric, as the variances are pair
wise symmetric. The sensitivity matrix S elements
are the sensitivities of the model output to changes
in particular parameters. The entries of the matrix P
are:
0)(det
T
T
21T2
T
)(det
)(adj
)(
SS
SS
SS
SSP
pp
xnn
(12)
When the matrix becomes singular: det(S
T
S)→0,
their entries are large and, therefore, associated error
estimates are large.
Even if structural identifiability of the model has
been previously confirmed, it may happen that the
matrix S
T
S is close to singular. Inversing such
matrix S
T
S cause very large entries of the matrix P,
which means a very large errors of parameter
estimates. Thus, the matrix S
T
S must be non-singular
and the sensitivity S large. Both, the sensitivity S
and the matrix S
T
S depend on the form of regression
function y
mod
. Choice of the regression function of
desired properties (large S and not singular, or not
close to singular, the matrix S
T
S) is the aim of our
investigation.
We assumed that model function is better than
the others if it gives lower objective function OF and
lower uncertainty ∆BV values. The smaller value of
OF, the better the function fit. According to the error
propagation rule, the smaller error pi of regression
function parameters the smaller ∆BV uncertainty of
BV. For example, when regression function depends
on two parameters p
1
and p
2
, then the uncertainty
∆BV is defined as follows (Ku, 1966):
21
2
21
2
2
2
2
2
1
2
1
pp
p
BV
p
BV
p
p
BV
p
p
BV
BV
(13)
where Δp
1
, Δp
2
are parameters p
1
and p
2
estimation
errors, Δp
1
p
2
is estimated covariance of p
1
and p
2
.
In literature it is presented opinion that the first
passage of tracer is asymmetrical, i.e. the rising and
Proposal of New Tracer Concentration Model in Lung PCT Study - Comparison with Commonly Used Gamma-variate Model
137
falling edges are not equally sloped (Lampaskis et
al., 2009; Thompson et al., 1964). In order to
examine symmetry between rising and falling edge
of concentration-time measurements in pCT lung
study, the edges were approximated by linear
function in the range of linear edge course. The
slope angles
R
and
F
were calculated. The
asymmetry coefficient
was defined as follows:
%100
R
FR
(14)
3 RESULTS
To compare the different regression functions 100
simulation runs were performed. For each simulation
run the value of objective function OF was
calculated for all compared regression functions.
Then, for each regression function the mean value of
the objective function was calculated. The results
obtained for AIF, vessels and parenchyma are
presented in table 1 - different regression functions
show different ability to fit measurements. Table 2
presents the mean BV and BV calculated according
to the error propagation rule, with respect to all
model parameters, for simulated data.
The value of BV, for AIF region and for all
regression functions, is known a priori and equal to
100, see table 2 and 4. It results from the equation 7:
the integrals in numerator and denominator of are
equal, so their quotient is equal to 1 and k
H
/ρ = 1. In
literature the BV is given in [ml/100g] therefore
equation 7 is multiplied by 100.
Table 1: Mean values of objective function for gamma-
variate, Gauss and Rayleigh functions in all regions
calculated for simulated data.
gamma-variate Gauss Rayleigh
AIF 9258 9198 9201
vessels 808 680 1218
parenchyma 193 186 230
Table 3 shows mean OF calculated for the actual
measurements taken from 5 subjects. Table 4 shows
example results of BV and ∆BV for a certain patient
calculated based on actual data.
Tables 5 shows rising and falling edge slope
angles
R
and
F
, their differences and asymmetry
coefficients
, separately for each of 5 subjects.
Table 6 shows mean values of rising and falling
edge slope angles
R
and
F
, their standard
deviations ∆
R
and ∆
F
, differences between mean
values of
R
and
F
and asymmetry coefficients .
Table 2: Mean BV parameters; their uncertainties ±∆BV
and CV calculated for simulated data, taking into account
errors p
i
of regression functions parameters p
i
.
BV [ml/100g]
±ΔBV [ml/100g]
CV [%]
gamma-variate Gauss Rayleigh
AIF
100
±0,2626
0,2626
100
±0,0760
0,0760
100
±0,2447
0,2447
vessels
19,1400
±61,5489
321,5700
19,9783
±0,0058
0,0290
21,0502
±0,0333
0,1582
parenchyma
6,1652
±0,0680
1,1030
6,2627
±0,0004
0,0064
5,6497
±0,0051
0,0903
Table 3: Mean values of objective function for gamma-
variate, Gauss and Rayleigh functions in all regions
calculated for 5 subjects, based on actual data.
gamma-variate Gauss Rayleigh
AIF 9411 7791 9129
vessels 853 706 1401
paren. 136 130 139
Table 4: Example results of BV parameter; its uncertainty
±∆BV and CV calculated for single subject (female, 63
years old), taking into account errors p
i
of regression
functions parameters p
i
, based on actual data.
BV [ml/100g]
±ΔBV [ml/100g]
CV [%]
gamma-variate Gauss Rayleigh
AIF 100
±0,1170
0,1170
100
±0,0311
0,0311
100
±0,2505
0,2505
vessels 10,3512
±20,2722
195,8440
8,5467
±0,0037
0,0433
11,0668
±0,0158
0,1428
parenchyma 8,4072
±0,0084
0,0999
8,1724
±0,0004
0,0049
8,3620
±0,0075
0,0897
Table 5: Rising and falling edge slope angles
R
and
F
,
their differences and asymmetry coefficients calculated
for 5 subjects, based on actual data.
R []
F [] |R|-|F| []
[%]
1
AIF 88,24 -88,70 -0,46 -0,52
vessels 87,26 -86,04 1,22 1,40
parenchyma 77,64 -75,41 2,23 2,87
2
AIF 86,71 -87,94 -1,23 -1,42
vessels 85,53 -84,56 0,97 1,13
parenchyma 74,86 -69,65 5,21 6,96
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
138
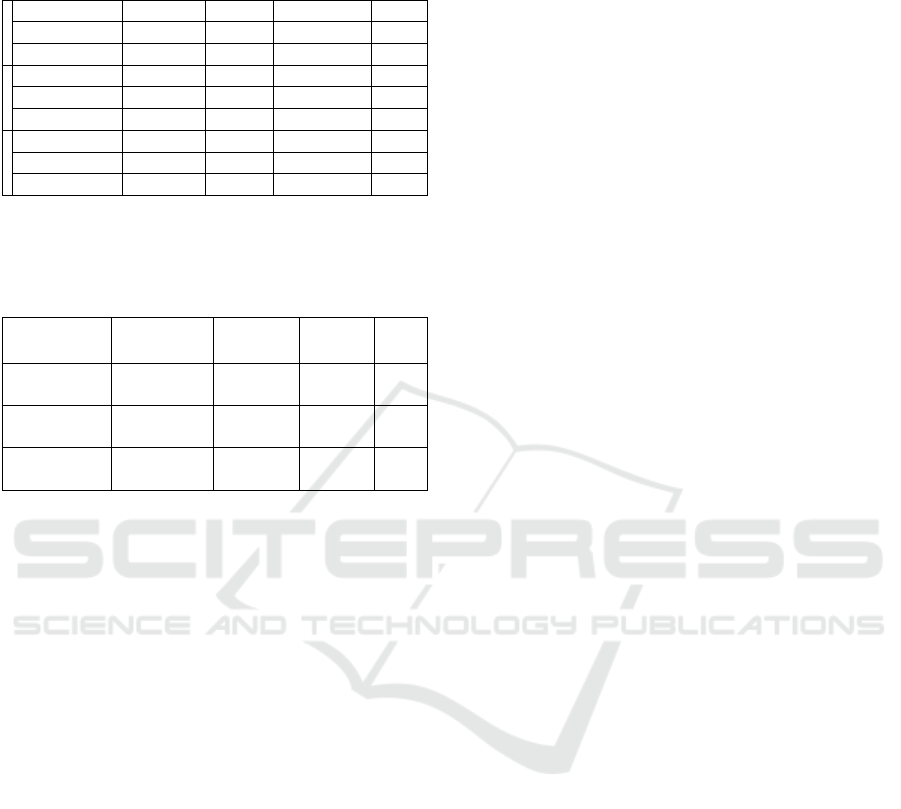
Table 5: Rising and falling edge slope angles
R
and
F
,
their differences and asymmetry coefficients calculate
d
for 5 subjects, based on actual data (cont.).
3
AIF 89,59 -89,02 0,57 0,64
vessels 85,37 -87,16 -1,79 -2,10
parenchyma 71,83 -73,00 -1,17 -2,57
4
AIF 88,41 -88,15 0,26 0,29
vessels 84,42 -75,40 9,02 10,69
parenchyma 79,22 -66,50 12,72 16,06
5
AIF 88,91 -88,96 -0,05 -0,06
vessels 86,40 -86,30 0,10 0,12
parenchyma 64,73 -66,50 -1,77 -2,73
Table 6: Mean values of rising and falling edge slope
angles
R
and
F
, their standard deviations
R
and
F
,
differences and asymmetry coefficients calculated for 5
subjects, based on actual data.
R []
±ΔR []
F []
±ΔF[]
|R|-
|F| []
[%]
AIF 88,37
±1,07
-88,55
±0,49
-0,18
-
0,21
vessels 85,80
±1,08
-83,89
±4,84
1,90 2,22
parenchyma 73,66
±5,73
-70,21
±3,96
3,44 4,68
4 DISCUSSION
Our aim is to determine which function best
approximates the first passage of tracer in pCT lung
studies. Fitting results, presented in table 1, show
different quality of fit for different regression
functions. Taking into account OF values, the Gauss
function proved to be the best in all considered
regions - AIF, vessels and parenchyma. Similar
conclusions can be drawn on the basis of uncertainty
analysis, see table 2. It is worth mentioning, that the
most frequently used gamma-variate function
produced noticeably higher BV than the Gauss and
the Rayleigh functions.
Simulated data analysis demonstrated the
advantage of the Gauss function over the other ones.
In order to confirm the simulation results, actual
measurements analysis was performed. The results,
presented in table 3, show that OF in all regions are
the lowest for the Gauss function. The Gauss
function best approximates the first passage of tracer
in AIF, blood vessels and parenchyma. Also, the
Gauss function produces the lowest uncertainty BV,
which means that the impact of model parameters
error on BV is the smallest.
It is widely accepted that the first passage of
tracer is asymmetrical, i.e. the rising and falling
edges are not equally sloped (Lampaskis et al., 2009;
Thompson et al., 1964). Considering this, regression
function that best approximates the first passage of
tracer should also be asymmetrical. However, it was
proved (table 1 and table 3) that the Gauss function,
which is symmetrical, best approximates the first
passage of tracer. The rising and falling edges of
concentration-time curves were approximated by the
linear function and slope angles were calculated.
Rising and falling edge slope angles, their
differences and asymmetry coefficients calculated
for 5 subjects are presented in table 5. Furthermore,
the mean values of slope angles, their standard
deviations, differences and asymmetry coefficients
were calculated and presented in table 6. Differences
between slope angles are insignificant and the
differences between mean values of rising and
falling edge slope angles, presented in table 6, are
negligible. Therefore, it can be assumed that rising
and falling edges of actual measurements are
symmetrical (very close to symmetrical). For that
reason, the Gauss function proved to be best
approximation of the first passage of tracer in pCT
lung studies.
5 CONCLUSIONS
This paper presents a comparative analysis of three
regression functions in three regions (AIF, blood
vessels and parenchyma) in pCT lung tests.
Considering results of simulated data analysis, the
Gauss function showed a clear advantage over the
others. Results of actual measurements analysis
confirmed that the Gauss function produce the most
accurate approximations of the first passage of
tracer. It was showed that contrary to the widely
accepted practice, the differences between rising and
falling edge slope angles of concentration-time
curves are inconsiderable. Therefore, one can
assume that rising and falling edges are symmetrical.
Negligible asymmetry of measurements justifies
why the Gauss function best approximates the first
passage of tracer in pCT lung studies.
ACKNOWLEDGEMENTS
This work was supported by funds of Faculty of
Electronics, Telecommunications and Informatics,
Gdańsk University of Technology.
Proposal of New Tracer Concentration Model in Lung PCT Study - Comparison with Commonly Used Gamma-variate Model
139

REFERENCES
Balthazar, E. (2011). CT Contrast Enhancement of the
Pancreas: Patterns of Enhancement, Pitfalls and
Clinical Implications. Pancreatology, 11(6), pp.585-
587.
Balvay, D., Ponvianne, Y., Claudon, M. and Cuenod, C.
(2008). Arterial input function: Relevance of eleven
analytical models in DCE-MRI studies. In:
Proceedings of 5th IEEE International Symposium on
Biomedical Imaging. Paris: IEEE.
Blomley, M. and Dawson, P. (1997). Bolus dynamics:
theoretical and experimental aspects. The British
Journal of Radiology, 70(832), pp.351-359.
Browarczyk, M., Kalicka, R. and Lipiński, S. (2015).
Lung Perfusion Parameters in the Diagnosis of
Diabetic Pulmonary Microangiopathy. In: I. Lacković
and D. Vasic, ed., 6th European Conference of the
International Federation for Medical and Biological
Engineering, 1st ed. Dubrovnik: Springer International
Publishing, pp.444-447.
Calamante, F., Thomas, D., Pell, G., Wiersma, J. and
Turner, R. (1999). Measuring Cerebral Blood Flow
Using Magnetic Resonance Imaging Techniques.
Journal of Cerebral Blood Flow & Metabolism,
pp.701-735.
Cao, Y. (2011). The Promise of Dynamic Contrast-
Enhanced Imaging in Radiation Therapy. Seminars in
Radiation Oncology, 21(2), pp.147-156.
Chen, M. and Siochi, R. (2011). Feasibility of using
respiratory correlated mega voltage cone beam
computed tomography to measure tumor motion.
Journal of Applied Clinical Medical Physics, 12(2),
p.3473.
Cobelli, C., Foster, D. and Toffolo, G. (2002). Tracer
kinetics in biomedical research. New York: Kluwer
Academic.
Cohen, M. (1966). The Organization of Clinical
Dosimetry: I. the four stages of clinical dosimetry.
Acta Radiologica: Therapy, Physics, Biology, 4(3),
pp.233-256.
Enderle, J., Blanchard, S. and Bronzino, J. (2000).
Introduction to biomedical engineering. San Diego:
Academic Press.
Hopkins, S., Henderson, A., Levin, D., Yamada, K., Arai,
T., Buxton, R. and Prisk, G. (2007). Vertical gradients
in regional lung density and perfusion in the supine
human lung: the Slinky effect. Journal of Applied
Physiology, 103(1), pp.240-248.
Jackson, A. (2004). Analysis of dynamic contrast
enhanced MRI. The British Journal of Radiology,
77(suppl_2), pp.S154-S166.
Kalicka, R. and Pietrenko-Dąbrowska, A. (2006).
Parametric Modeling of DSC-MRI Data with
Stochastic Filtration and Optimal Input Design Versus
Non-Parametric Modeling. Annals of Biomedical
Engineering, 35(3), pp.453-464.
Kalicka, R., Browarczyk, M. and Lipiński, S. (2015).
Usefulness of chest perfusion computed tomography
in the diagnosis of diabetic pulmonary
microangiopathy. Biocybernetics and Biomedical
Engineering, 35(1), pp.68-73.
Ku, H. (1966). Notes on the use of propagation of error
formulas. Journal of Research of the National Bureau
of Standards, Section C: Engineering and
Instrumentation, 70C(4), p.263.
Lampaskis, M., Strouthos, C. and Averkiou, M. (2009).
Application of tracer dilution models for the
quantification of perfusion with contrast enhanced
ultrasound imaging. In: Proceedings of 14th European
Symposium on Ultrasound Contrast Imaging.
Rotterdam.
Lilienfield, L., Kovach, R., Marks, P., Hershenson, L.,
Rodnan, G., Ebaugh, F. and Freis, E. (1956). THE
HEMATOCRIT OF THE LESSER CIRCULATION
IN MAN 12. Journal of Clinical Investigation, 35(12),
pp.1385-1392.
Mírka, H., Ferda, J., Baxa, J., Třeška, V., Liška, V.,
Schmidt, B. and Flohr, T. (2010). Perfusion CT of the
liver. Czech Radiology, 64(4), pp.281-289.
Nakano, S., Gibo, J., Fukushima, Y., Kaira, K., Sunaga,
N., Taketomi-Takahashi, A., Tsushima, Y. and Mori,
M. (2013). Perfusion Evaluation of Lung Cancer.
Journal of Thoracic Imaging, 28(4), pp.253-262.
Ng, Q. and Goh, V. (2010). Angiogenesis in Non-small
Cell Lung Cancer. Journal of Thoracic Imaging,
25(2), pp.142-150.
Pevsner, A., Nehmeh, S., Humm, J., Mageras, G. and Erdi,
Y. (2005). Effect of motion on tracer activity
determination in CT attenuation corrected PET images:
A lung phantom study. Med. Phys., 32(7), p.2358.
Praveenkumar, R., Augustine, A. and Santhosh, K. (2011).
Estimation of inhomogenity correction factors for a
Co-60 beam using Monte Carlo simulation. Journal of
Cancer Research and Therapeutics, 7(3), p.308.
Sauter, A., Feldmann, S., Spira, D., Schulze, M., Klotz, E.,
Vogel, W., Claussen, C. and Horger, M. (2012).
Assessment of Splenic Perfusion in Patients with
Malignant Hematologic Diseases and Spleen
Involvement, Liver Cirrhosis and Controls Using
Volume Perfusion CT (VPCT). Academic Radiology,
19(5), pp.579-587.
Semmlow, J. (2005). Circuits, signals, and systems for
bioengineers. Oxford: Academic.
Srikanchana, R., Thomasson, D., Choyke, P. and Dwyer,
A. (2004). A comparison of pharmacokinetic models
of dynamic contrast enhanced MRI. In: Proc. IEEE
Symp. Computer-Based Med. Syst.. Bethesda: IEEE,
pp.361-366.
Thompson, H., Starmer, C., Whalen, R. and Mcintosh, H.
(1964). Indicator Transit Time Considered as a Gamma
Variate. Circulation Research, 14(6), pp.502-515.
Wintermark, M., Sincic, R., Sridhar, D. and Chien, J.
(2008). Cerebral perfusion CT: Technique and clinical
applications. Journal of Neuroradiology, 35(5),
pp.253-260.
Zhao, H., Gong, J., Wang, Y., Zhang, Z. and Qin, P.
(2010). Renal hemodynamic changes with aging: a
preliminary study using CT perfusion in the healthy
elderly.
Clinical Imaging, 34(4), pp.247-250.
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
140
