
Wearable Motion Tolerant PPG Sensor for Instant Heart Rate in
Daily Activity
Takanori Ishikawa
1
, Yasuhide Hyodo
1
, Ken Miyashita
2
, Kazunari Yoshifuji
1
,
Yota Komoriya
1
and Yutaka Imai
1
1
Interface Device Development Dept., UI Device Development Div., Device & Material R&D Group, R&D Platform,
Sony Corporation, 4-14-1 Asahi-cho, 243-0014, Atsugi-shi, Kanagawa, Japan
2
Intelligent Application Technology Development Dept., Application Technology Development Div., System R&D Group,
R&D Platform, Sony Corporation, 2-10-1 Osaki, 141-8610, Shinagawa-ku, Tokyo, Japan
Keywords: PPG Sensor, Motion Artifact Cancellation Framework, Heart Rate Variability, Daily Activity, Ambulatory.
Abstract: A wristband-type PPG heart rate sensor capable of overcoming motion artifacts in daily activity and
detecting heart rate variability has been developed together with a motion artifact cancellation framework.
In this work, a motion artifact model in daily life was derived and motion artifacts caused by activity of arm,
finger, and wrist were cancelled significantly. Highly reliable instant heart rate detection with high noise-
resistance was achieved from noise-reduced pulse signals based on peak-detection and autocorrelation
methods. The wristband-type PPG heart rate sensor with our motion artifact cancellation framework was
compared with ECG instant heart rate measurement in both laboratory and office environments. In a
laboratory environment, mean reliability (percentage of time within 10% error relative to ECG instant heart
rate) was 86.5% and the one-day pulse-accuracy achievement rate based on time use data of body motions
in daily life was 88.1% or approximately 21 hours. Our device and motion artifact cancellation framework
enable continuous heart rate variability monitoring in daily life and could be applied to heart rate variability
analysis and emotion recognition.
1 INTRODUCTION
A variety of applications using wristband-type,
photoplethysmography (PPG)-based heart rate
sensors have been proposed in recent years. Their
typical applications are heart rate monitoring in a
resting state for healthcare, feedback to efficient
training methods in walking and running state as
well as estimation of consumed calories. These
applications are based on averaged heart rate to
reduce motion artifact.
Though accurate monitoring of heart rate
variability (HRV) has not yet been achieved in
motion state, once it is achieved, it will open a
variety of applications including like music
recommendation based on user affective state, stress
monitoring and cardiac insufficiency detection
(Wijsman, 2013); (Shin, 2014); (Venema, 2015).
In comparison with chest-strap-type wearable
electrocardiography (ECG) monitors, wristband-type
PPG heart rate sensors can be comfortably worn
without placing a burden on the user, which gives
them a user-friendly advantage. On the other hand,
wristband-type PPG sensors suffer from a
superimposing of pseudo pulse signals (motion
artifacts) caused by user motion, which makes
accurate calculation of heart rate difficult (Tamura,
2014). Consequently, to accurately calculate pulse
rate from pulse signals superimposed with motion
artifacts, various types of motion artifact reduction
methods have been proposed to improve accuracy of
HRV from PPG raw data(Renevey, 2001); (Asada,
2004), and there have been many studies targeting
periodic motion artifacts caused by intense arm
motion while walking or running. So far many
studies have reported evaluation average pulse rate
using average heart rate by ECG as reference.
Focusing on motion in daily life, we have to take
into account random motion of arm, finger and wrist.
An experimental protocol was proposed that
includes activity of both arm, finger and wrist (Parak,
2014); (Binsch, 2016); (Tăuţan, 2015), but their
study was limited to evaluation and analysis of
motion artifact.
126
Ishikawa T., Hyodo Y., Miyashita K., Yoshifuji K., Komoriya Y. and Imai Y.
Wearable Motion Tolerant PPG Sensor for Instant Heart Rate in Daily Activity.
DOI: 10.5220/0006109901260133
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 126-133
ISBN: 978-989-758-212-7
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

In this paper, we report on a motion artifact
cancellation framework that we have developed
based on the results of analyzing arm and finger
motion artifacts assuming motions in daily life. We
also report on laboratory and ambulatory evaluation
of this framework using instant heart rate by ECG as
reference with our design of experimental protocols
simulating motions in daily life.
2 PROBLEM STATEMENT
2.1 Photoplethysmography
The reflective PPG method measures changes in
blood flow in subcutaneously distributed capillaries
by injecting light from a light source into the skin
and measuring the intensity of returning light by a
receiver after absorption and diffusion by blood flow
and skin tissue several mm under the skin (Renevey,
2001).
∙
∙
(1)
Here,
is the intensity of the light incident on the
skin,
is the temporal change in diffusion
and absorption by hemoglobin, etc. in capillary
blood flow,
is the amount of diffusion and
absorption by body tissue, and
is the intensity
of reflected light.
2.2 Motion Artifact
There are activities of arm, finger and wrist in daily
life affecting wristband-type PPG sensors. In the
case of finger and wrist motions, the muscles under
the wrist move causing the state of blood flow in
subcutaneous body tissue to change. This would
mean that
in Eq. (1) is not fixed. We
therefore performed an analysis of motion artifacts
caused by arm and finger motion in the following
way.
While the thickness of each layer under the skin
differs by site, age, etc., body tissue lies at a depth of
1.0 – 2.0 mm (Boucsein, 2012). To measure the state
of blood flow in subcutaneous tissue in a non-
invasive manner, we adopted the PPG method.
Taking into account the subcutaneous penetration of
light (Bashkatov, 2005) and the optical absorption
and diffusion coefficients of hemoglobin, we
determined an optimal wavelength as follows. First,
we analyzed the optical wavelength dependency of
the signal-to-noise ratio (S/N) by measuring the
pulse signal when bending and stretching the index
finger periodically (2 Hz) and performing a
frequency analysis. In calculating S/N, we took the
heart-rate band as the signal band and defined the
finger-motion frequency band as the noise band.
Table 1: LED wavelength dependency of S/N when
bending and stretching the index finger periodically.
Table 1 lists the wavelength dependency of S/N
when bending and stretching the index finger
periodically as measured with three subjects. The
wavelengths used in this analysis are those of easily
obtainable LEDs. These results show that S/N is
smallest for a wavelength of 630 nm, so we chose it
to be the optical wavelength of the PPG signal that
reflects finger motion well.
However, for the pulse signal, we chose a
wavelength of 530 nm to measure the state of blood
flow in the capillaries of the dermic layer that is not
easily affected by body tissue (Faber, 2004); (Lee,
2013). Here, we simultaneously measured the arm’s
acceleration signal by having a 3-axis acceleration
sensor worn on the wrist.
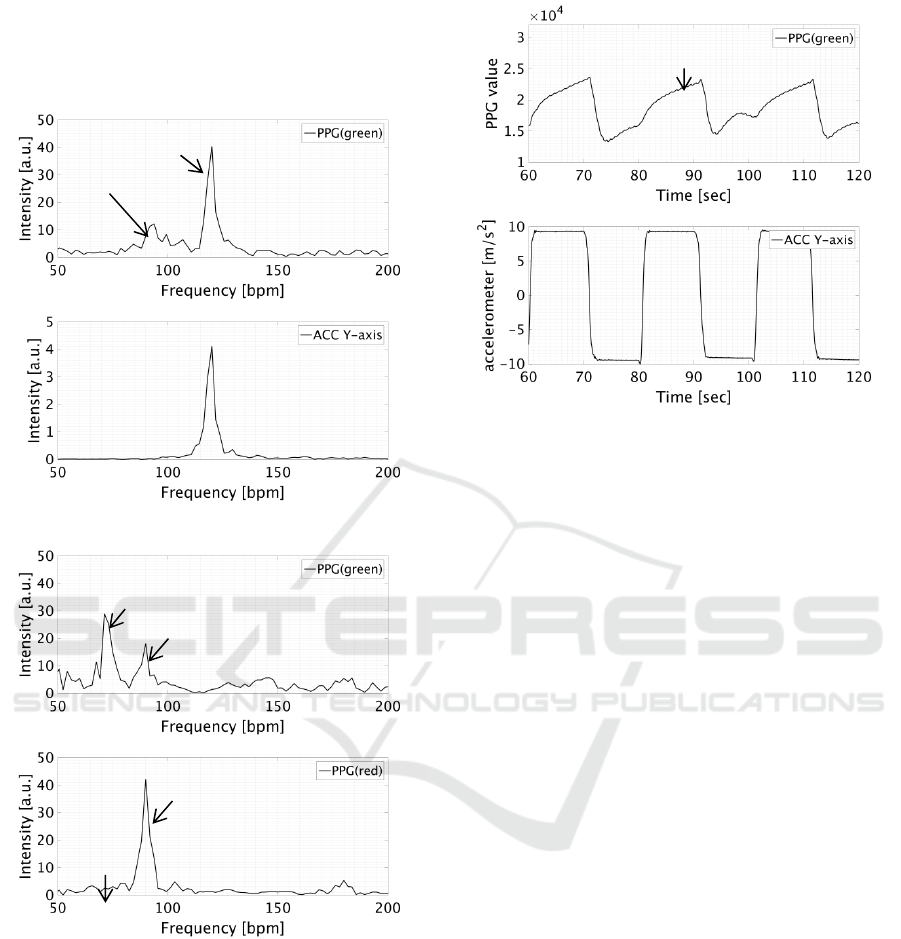
Figure 2 (A) shows the results of a PPG (green)
and acceleration frequency analysis when moving
the arm back and forth with a 2 Hz period in a
standing position. Strong peaks are observed in the
PPG (green) spectral distribution near the arm-
moving frequency of 120 [bpm] and the heartbeat
frequency of 100 [bpm]. This shows that the arm-
related motion artifact is superimposed as a pseudo
pulse signal. Since a motion artifact is easily
superimposed when blood is flowing, it is thought
that body motion affects blood flow and a pseudo
pulse signal arises due to pseudo blood flow.
Figure 2 (B) shows the results of a PPG (green)
and a PPG (red) frequency analysis when bending
and stretching the index finger periodically at 1.5 Hz
while keeping the arm fixed in a sitting position.
Although the acceleration sensor measured no finger
motion, a strong peak is observed near the finger-
moving frequency of 90 [bpm] in the PPG (green)
and PPG (red) spectral distributions. In particular,
the PPG (red) spectral distribution shows that the
spectral intensity at the motion artifact frequency of
90 [bpm] is stronger than that at the heartbeat
frequency of 60 [bpm] compared with PPG (green).
This is thought to be that the hemoglobin diffusion
coefficient is smaller and the degree of subcutaneous
penetration deeper at 630 nm than at 530 nm.
Therefore, we suppose that intensity of returning
Subject 470nm 530nm 630nm 860nm 940nm
#1 1.90 7.75 -7.22 -5.66 -5.75
#2 2.25 7.61 -18.30 -10.20 -15.47
#3 7.72 4.67 -13.02 -7.95 2.83
Wearable Motion Tolerant PPG Sensor for Instant Heart Rate in Daily Activity
127
light from skin is smaller compares with wavelength
of 530nm. Furthermore, we have observed that
motion artifact caused by wrist motion has same
characteristic in frequency analysis.
Figure 3 shows the results of measuring PPG (green)
and acceleration signal in the fingertip direction
when moving the arm up and down relative to the
heart in a standing position. When the arm is moved
above the heart, the total amount of blood flow
decreases due to the effect of gravity. Therefore,
baseline of the intensity of return light decreases.
Figure 3: Up-and-down arm motion relative to heart.
Upper: PPG (green), lower: acceleration signal.
Conversely, when moving the arm below the heart,
it can be seen that this baseline increases. Since the
baseline stabilizes in approximately two seconds
after lifting the arm, noise frequency is assumed to
be under 0.5 Hz. The above experimental results
show that the intensity of return light from skin is
affected by activity of arm, finger and wrist. We can
therefore extend Eq. (1) as follows.
∙
∙
∙
∙
(2)
Here,
is the amount of optical absorption
and diffusion in body tissue due to finger motion,
is the amount of optical absorption and
diffusion due to changes in blood flow caused by
arm motion, and
is the amount of optical
absorption and diffusion due to the arm’s up or
down orientation. If we now take the logarithm of
both sides of this equation, Eq. (2) can be rewritten
as follows.
(3)
Here,
is the pulse signal,
is the noise
signal due to finger motion,
is the noise signal
due to arm motion, and
is the noise signal due
to arm orientation. In other words, this equation
shows that all motion artifacts in the PPG method
are superimposed on the observed signal.
(A) When moving the arm back and forth at 2 Hz
(B) When bending and stretching the index finger at 1.5
Hz
Figure 2: Results of frequency analysis. (A) upper: PPG
(green), lower: acceleration signal (Y-axis means fingertip
direction); (B) upper: PPG (green), lower: PPG (red).
pulse
down
up
Pulserate
Motionartifact
Pulserate
Motionartifact
Motionartifact
Pulserate
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
128
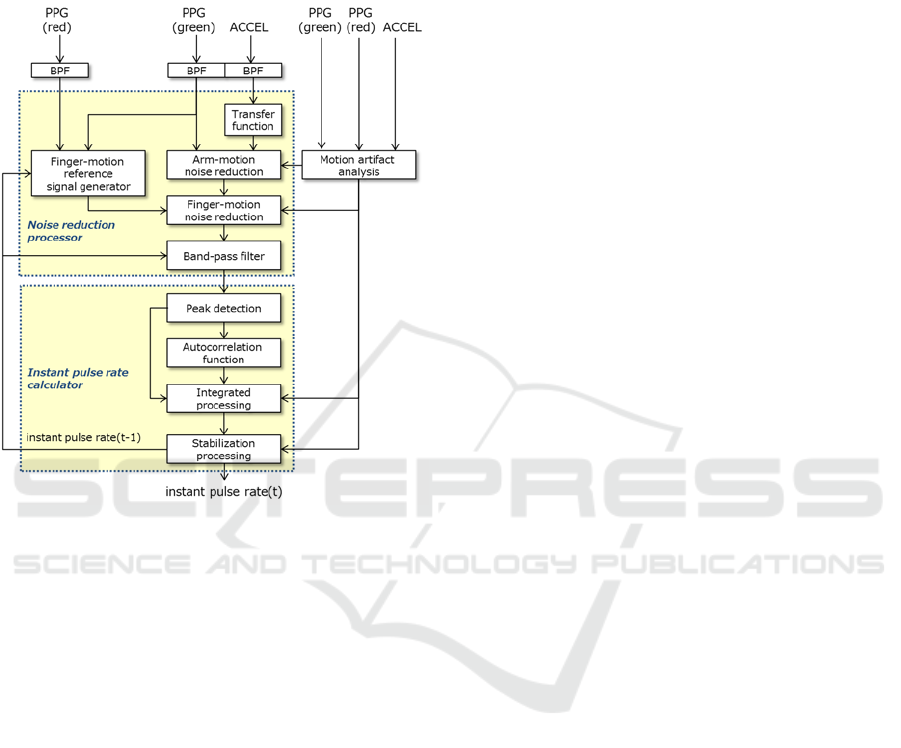
3 PROPOSED METHOD
A motion artifact cancellation framework based on
Eq. (3) that assumes an additive model for arm and
finger motion artifacts is shown in Figure 4.
Figure 4: Schematic diagram of motion artifac
t
cancellation framework.
To first step in this framework is to separate the
motion artifact due to the orientation of the arm
using a band-pass filter (BPF). Here, the pass band is
set to [0.5, 5] Hz taking into account the arm-
orientation noise band and the heart-rate frequency
band.
The next step is to separate arm-motion noise by
an adaptive filter using the acceleration signal as a
reference signal. It is important in noise reduction
processing using an adaptive filter to select a
reference signal having high correlation with the
noise signal superimposed on the observed signal
(Asada, 2004). For this reason, we modelled
beforehand the transfer function to body-motion
blood flow as a finite impulse response (FIR) filter
through system identification. The signal resulting
from the convolution of the acceleration signal with
the FIR filter is used as a reference signal for arm-
motion noise reduction processing.
Similarly, finger-motion noise is separated using
a reference signal. Here, we use the PPG signal as a
reference signal to make non-invasive measurements
of the state of blood flow in body tissue. We decided
that an optical wavelength is 630 nm based on
previous analysis results in Table 1.
However, a pulse signal component is also
included in PPG (red). To therefore make correlation
with the noise signal even higher, we use a BPF to
weaken the pulse signal component based on the
finally estimated heart rate. Furthermore, assuming
use in ambulatory environment and knowing that
noise will also be generated by changes in contact
pressure caused by deformation of the arm’s shape,
we also use a BPF to reduce this noise.
Next is the calculation of the instant pulse rate
from the pulse signal reduced of motion artifacts.
Although peak detection is applied here to calculate
the instant pulse rate, erroneous detections can easily
occur due to the effects of residual noise. To deal
with this noise, we introduce pulse rate detection by
an autocorrelation function exploiting the periodicity
of the pulse signal. Pulse rate calculation by an
autocorrelation function calculates the inter-beat-
interval (IBI) from the lag at which the correlation
function becomes maximum. However, while the
autocorrelation method is highly robust to noise, its
assumption of periodicity in the pulse signal can
result in lower accuracy than the peak detection
method if pulse variability are present. For this
reason, the integrated processing section in this
framework first calculates the instant pulse rate by
both the peak detection method and autocorrelation
method and the reliability of each result. It then
outputs the optimal instant pulse rate based on the
pulse signal and body motion information analyzed
from the acceleration signal.
4 EXPERIMENTAL VALIDATION
4.1 Heart Rate Sensor Prototype
Our prototype for a wristband PPG heart rate sensor
is shown in Figure 5. This device performs pulse
signal measurement by irradiating the human body
with green LED light and measuring reflected light
with a photodetector. It also performs reference-
signal measurement in the finger-motion noise-
reduction process by measuring reflected light from
a red LED likewise with a photodetector. In addition,
it obtains a reference signal in the arm-motion noise-
reduction process by measuring arm acceleration
with a 3-axis acceleration sensor built into the sensor
enclosure. The pulse signal obtained from measured
green-LED and red-LED light and the acceleration
signal are recorded in built-in eMMC flash storage.
The sampling frequency is 128 Hz for all sensors.
Wearable Motion Tolerant PPG Sensor for Instant Heart Rate in Daily Activity
129
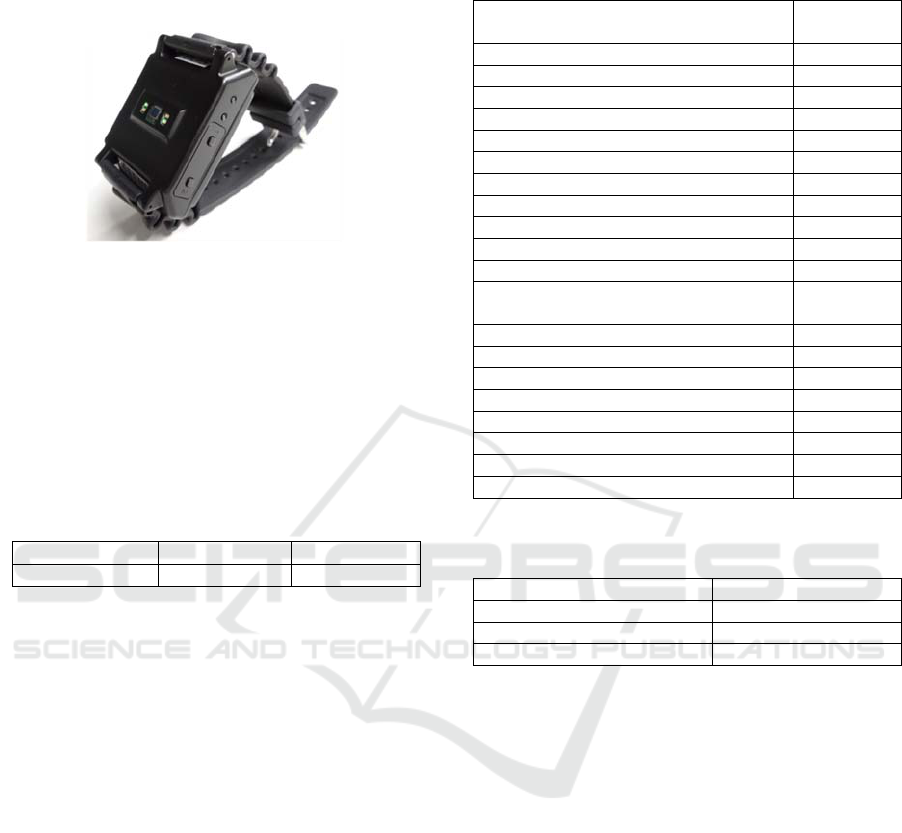
The device incorporates a real-time clock (RTC) that
can be synchronized with a host PC and recorded
together with measured data.
Figure 5: Prototype of wristband type heart rate sensor.
4.2 Subjects
The experiment was conducted with 12 subjects
recruited from male employees in the workplace.
After obtaining approval from the Sony Life Ethics
Committee, the subjects were briefed about the
contents of this research project and their consent
was obtained in writing.
Table 2: Subjects.
4.3 Laboratory Protocol and
Evaluation
Evaluating motion artifacts in daily life requires
knowledge of user lifestyle for users wearing a
wristband-type device. Although there are various
types of user lifestyles, activity time use by types of
behavior for one week can be summarized as
follows according to material from the Statistics
Bureau of the Ministry of Internal Affairs and
Communications (MIC) (Ministry of Internal Affairs
and Communications, 2011). However, an
evaluation based on protocol that mimics these
activities as-is puts an unrealistic burden on subjects,
so we designed original protocols after reclassifying
activities into three main states of body motion.
These are (1) active state featuring periodic arm
motion of fixed intensity as in walking when
commuting to school or work or engaging in sports
(such as jogging or running), (2) semi-resting state
featuring non-periodic arm motion of random
intensity and finger activity as in schoolwork, work,
housework, meals, etc. and (3) resting state as in
sleeping. The results of reorganizing the above
activities are listed on Table 4.
Specifically, we designed three types of protocols
based on the above results of reclassifying activity
time use by types of behavior. First, to evaluate
periodic motion artifacts in an active state, we
designed a “run” protocol which is taken into
account for walking when commuting to school or
work or engaging in sports such as jogging or
running. Next, to evaluate non-periodic motion
artifacts in a semi-resting state, we designed “daily1”
and “daily2” protocols which are taken account for
habitual tasks in daily life and business-related work,
schoolwork, housework, etc. For each protocol, the
subject began by resting in a sitting position to
condition his heart rate. All three protocols were not
performed on the same day to lighten the load on
subjects.
We used the Shimmer3 ECG unit from Shimmer
to provide a reference measurement for instant heart
rate. The sampling frequency is 512 Hz. Electrode
positioning was also optimized for each subject to
enhance S/N of the ECG signal. The instant heart
rate was calculated from the time intervals of the R
Characteristic
μσ
Range
Age
35.00 6.34
26 – 43
Table 3: Detailed activity time use by types of behavior
for one week.
Activity
Duration
(hh:mm)
Sleep 07:42
Personal care 01:19
Meals 01:39
Commuting to and from school or work 00:31
Work 03:33
Schoolwork 00:39
Housework 01:27
Caring or nursing 00:03
Child care 00:14
Shopping 00:26
Moving (excluding commuting) 00:30
Watching TV, listening to the radio,
reading a newspaper or magazine
02:27
Rest and relaxation 01:31
Learning, self-education, and training 00:12
Hobbies and amusements 00:44
Sports 00:14
Volunteer and social activities 00:04
Social life 00:19
Medical examination or treatment 00:08
Other activities 00:17
Table 4: Results of reclassifying activity time use by types
of behavior.
Class Duration (%)
Resting state 32.8
Semi-resting state 65.1
Active state 3.1
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
130

wave in the ECG signal.
The subject wore the prototype PPG sensor on
his non-dominant hand, and time synchronization
with shimmer3 was achieved through time
synchronization between the prototype device and
the host PC.
In comparing the instant heart rate calculated
from ECG and the instant pulse rate calculated from
the prototype device, we used resampled values at 1
Hz by linear interpolation for each. Furthermore, in
performing a quantitative evaluation of the accuracy
of detecting heart rate variability, we defined
reliability and accuracy as follows (Delgado-
Gonzalo, 2014).
Reliability is defined as the percentage number
of samples for which error is within 10% of the ECG
instant heart rate. Accuracy, meanwhile, is the mean
complement of error with the ECG instant heart rate.
Table 8 lists mean reliability and accuracy across
all subjects for each protocol in a laboratory
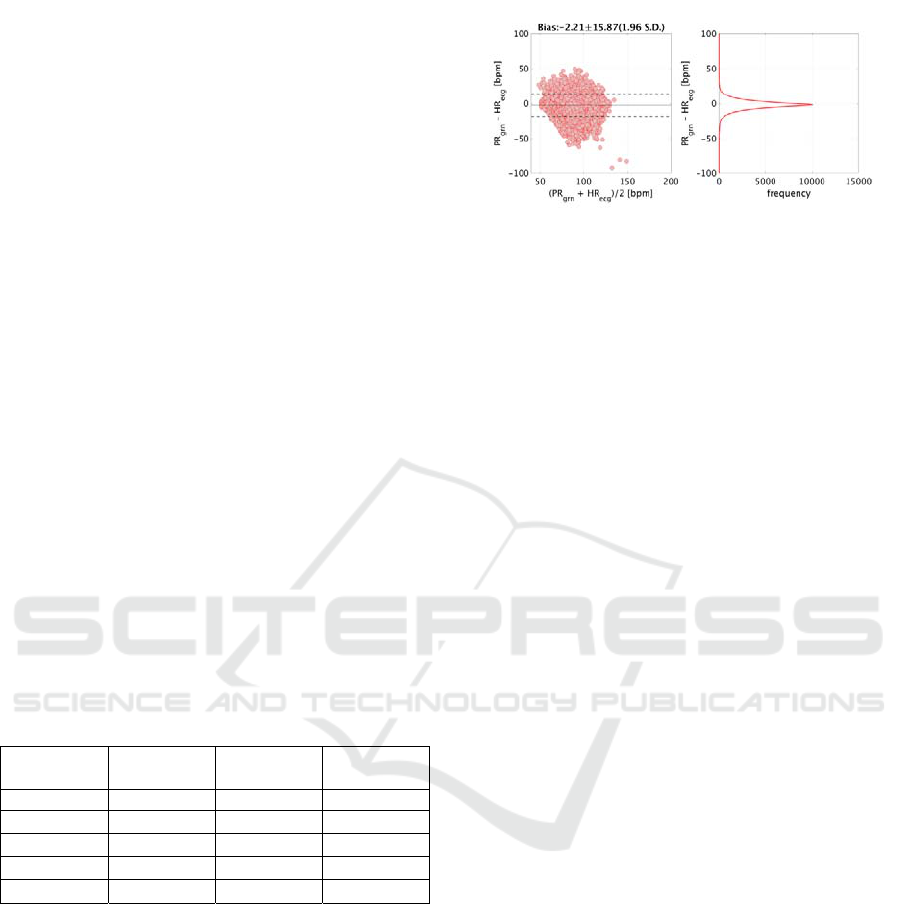
environment. Figure 6 shows Bland-Altman plots of
the ECG instant heart rate and PPG instant pulse rate
for each experimental protocol.
Based on the quantitative evaluation results for each
experimental protocol of Table 8 and activity time
use by types of behavior of Table 4, we estimated
the percentage of measurements within 10% of the
ECG heart rate for one day (referred to below as
“pulse-accuracy achievement rate”) in the following
way. Reliability of resting state was taken to be
reliability in a resting state over the run protocol,
daily1 protocol, and daily2 protocol. As a result,
subject mean of them was 97.5%. Next, reliability of
active state was taken to be reliability in the range
Table 5: Run protocol.
Task
Duration
(min)
Take deep breaths sitting down 2
Stand and rest 1
Walk on treadmill at 5 km/h 3
Walk on treadmill at 7 km/h 3
Walk on treadmill at 11 km/h 3
Take a break standing up 2
Table 6: Daily1 protocol.
Task
Duration
(min)
Take a break sitting down 2
Take deep breaths sitting down 2
Gesture that is supposed personal care 2
Gesture that is supposed meals 2
PC operation that is supposed office work 2
Smart phone operation 2
Gesture that is supposed housework 2
Take a break sitting down 2
Table 7: Daily2 protocol.
Task
Duration
(min)
Take a break sitting down 2
Gesture that is supposed reading book 2
Gesture that is supposed writing down 2
Gesture that is supposed conversation 2
Take a break sitting down 2
Table 8: Subject mean of reliability and accuracy of
estimated instant heart rate for each protocol in a
laboratory environment (*Accuracy = 100 - mean
percentage error).
Protocol Reliability (%) *Accuracy (%)
Run protocol 83.7 93.9
Daily1 protocol 82.7 93.8
Daily2 protocol 93.0 96.1
Mean 86.5 94.6
Figure 6: Results of Bland-Altman analysis of ECG instan
t
heart rate and PPG instant pulse rate. Top: run protocol;
middle: daily1 protocol; bottom: daily2 protocol. HR
ecg
and PR
grn
denote ECG instant heart rate and PPG instan
t
pulse rate, respectively.
Wearable Motion Tolerant PPG Sensor for Instant Heart Rate in Daily Activity
131
from standing to running tabulated for the run
protocol. As a result, subject mean of reliability was
81.4%. Finally, reliability of semi-resting state was
taken to be reliability outside the resting state
tabulated over the daily1 protocol and daily2
protocol. As a result, subject mean of them was
83.7%. Based on the above results, the day pulse-
accuracy achievement rate turned out to be 88.1% or
21.1 hours when converted to time.
5 DISCUSSION
We evaluated the reliability and accuracy of the
prototype wristband-type PPG sensor and motion
artifact cancellation framework in ambulatory
environment. In the experiment, we randomly
selected 4 of the 12 subjects and took pulse
measurements during their working hours. Table 9
lists reliability and accuracy values for each of these
subjects. Body motions from the subjects’ activity
records were typically computer keyboard
operations, note-taking/writing, lunch, and
smartphone use during breaks, which means
activities near those of the daily1 and daily2
protocols. However, reliability in an office
environment was found to be 77.9% or about 10%
lower than the 87.9% average reliability of the
daily1 and daily2 protocols.
Table 9: Subject mean of reliability and accuracy of
instant heart rate estimation in an office environment.
Subject
Duration
(hh:mm)
Reliability
(%)
Accuracy
(%)
#1 08:00 68.7 91.4
#2
08:00
84.1 94.3
#3
08:00
77.9 93.3
#4
08:00
80.9 93.8
Mean (N=4)
08:00
77.9 93.2
Figure 7 shows Bland-Altman plots of the ECG
instant heart rate and PPG instant pulse rate.
Compared with the Bland-Altman plots for the
daily1 and daily2 protocols of Figure 6, the heart
rate band has broadened and estimation error of the
instant heart rate has increased. This broadening of
the heart rate band can be explained as follows. For
the daily1 and daily2 protocols in a laboratory
environment, subjects carried out their tasks in a
sitting position, but in the office-environment
experiment, their heart rates would increase as they
walked to conference rooms or cafeterias or engaged
in discussions during meetings. Next, the increase in
error is thought to be due to changes in contact
Figure 7: Results of Bland-Altman analysis of ECG instan
t
heart rate and PPG instant pulse rate in an office
environment. HR
ecg
and PR
grn
denote ECG instant heart
rate and PPG instant pulse rate, respectively.
pressure between the pulse sensor and subject’s
body due to deformation of the arm’s shape caused
by motion or twisting of the wrist. If motion artifacts
due to changes in contact pressure can be formalized
as an additive model, our proposed framework
should be able to incorporate them.
6 CONCLUSIONS
We proposed a motion artifact cancellation
framework for a wristband-type heart rate sensor. As
part of this framework, we derived a motion artifact
additive model based on the results of motion
artifact analysis.
First, to cancel arm-related motion artifacts, we
modelled the transfer function to blood flow as an
FIR filter through system identification and used the
signal resulting from convolution of the acceleration
signal as a reference signal to improve the arm-
related motion artifact reduction effect. Next, to
cancel finger and wrist-related motion artifacts, we
measured blood flow in body tissue by PPG at 630
nm, weakened the pulse-signal component in that
signal by a band-pass filter based on heart rate, and
used the result as a reference signal to improve the
finger-related motion artifact reduction effect.
Finally, for pulse rate calculation, we integrated
the results of calculating pulse rate with reliability
by both the peak-detection and autocorrelation
methods thereby achieving instant pulse rate
detection with high noise resistance and high
accuracy. The pulse-measurement accuracy-
achievement rate was estimated to be 88.1% or 21.1
hours, which indicates that the prototype device and
motion artifact cancellation framework can detect
variability in heart rate with high accuracy in daily
activity.
BIOSIGNALS 2017 - 10th International Conference on Bio-inspired Systems and Signal Processing
132

REFERENCES
Wijsman, J., Vullers, R., Polito, S., Agell, C., Penders, J.,
Hermens, H., 2013. Towards Ambulatory Mental
Stress Measurement from Physiological Parameters. In
Affective Computing and Intelligent Interaction, pp.
564 - 569.
Shin, I. H., Cha, J., Cheon, G. W., Lee. C., Lee, S. Y.,
Yoon, H. J., Kim, H. C., 2014. Automatic stress-
relieving music recommendation system based on
photoplethysmography-derived heart rate variability
analysis. In 36th Annual International Conference of
the IEEE Engineering in Medicine and Biology
Society, pp. 6402-6405.
Venema, B., Blazek, V., Leonhardt, S., 2015. In-Ear
Photoplethysmography for Mobile Cardiorespiratory
Monitoring and Alarming. In IEEE 12th International
Conference on Wearable and Implantable Body Sensor
Networks, pp. 1-5.
Tamura, T., Maeda, Y., Sekine M. and Yoshida M., 2014.
Wearable Photoplethysmographic Sensors—Past and
Present. In Electronics, vol. 3, pp. 282-302.
Renevey, P., Vetter, R., Krauss, J., Celka, P., Depeursinge,
Y., 2001. Wrist-located pulse detection using IR
signals, activity and nonlinear artifact cancellation. In
Engineering in Medicine and Biology Society,
Proceedings of the 23rd Annual International
Conference of the IEEE, vol. 3, pp. 3030-3033.
Asada, H. H., Jiang, H. H., Gibbs, P., 2004. Active noise
cancellation using MEMS accelerometers for motion-
tolerant wearable bio-sensors. In Engineering in
Medicine and Biology Society, 26th Annual
International Conference of the IEEE, vol. 1, pp.
2157-2160.
Parak, J., Korhonen, I., 2014. Evaluation of wearable
consumer heart rate monitors based on
photopletysmography. In 36th Annual International
Conference of the IEEE Engineering in Medicine and
Biology Society, pp. 3670-3673.
Binsch, O., Wabeke, T., Valk, P., 2016. Comparison of
three different physiological wristband sensor systems
and their applicability for resilience- and work load
monitoring. In 13th International Conference on
Wearable and Implantable Body Sensor Networks, pp.
272-276.
Tăuţan, A. M., Young, A., Wentink, E., Wieringa, F.,
2015. Characterization and reduction of motion
artifacts in photoplethysmographic signals from a
wrist-worn device. In 37th Annual International
Conference of the IEEE Engineering in Medicine and
Biology Society, pp. 6146-6149.
Ministry of Internal Affairs and Communications,
Statistics Bureau, 2011. Basic survey on social life.
http://www.stat.go.jp/data/shakai/2011/pdf/gaiyou2.pdf.
Boucseinm W., 2012. Electrodermal Activity, Springer.
2nd edtion.
Bashkatov, A. N., Genina, E. A., Kochubey, V. I. and
Tuchin, V. V., 2005. Optical properties of human skin,
subcutaneous and mucous tissues in the wavelength
range from 400 to 2000 nm. In Applied Physics, vol.
38, number 15.
Faber, D. J., Aalders, M. C. G., Mik, E. G., Hooper, B. A.,
Gemert, M. J. C., Leeuwen, T. G., 2004. Oxygen
Saturation-Dependent Absorption and Scattering of
Blood. In PHYSICAL REVIEW LETTERS, vol. 93,
number 2.
Lee, J., Matsumura, K., Yamakoshi, K., Rolfe, P., Tanaka,
S., Yamakoshi, T., 2013. Comparison between red,
green and blue light reflection photoplethysmography
for heart rate monitoring during motion. In 35th
Annual International Conference of the IEEE
Engineering in Medicine and Biology Society, pp.
1724-1727.
Delgado-Gonzalo, R., Parak, J., Tarniceriu, A., Renevey,
P., Bertschi, M., Korhonen, I., 2014. Evaluation of
accuracy and reliability of PulseOn optical heart rate
monitoring device. In 37th Annual International
Conference of the IEEE Engineering in Medicine and
Biology Society, pp. 430-433.
Wearable Motion Tolerant PPG Sensor for Instant Heart Rate in Daily Activity
133
