
Attachable Micro-endoscopy System to Conventional Microscope for
Live Mouse Organ Imaging using 4f Configuration
Yoon Sung Bae
1,2
, Jae Young Kim
3
and Jun Ki Kim
1,2
1
Biomedical Engineering Research Center, Asan Institute for Life Science, Asan Medical Center, Seoul, Korea
2
University of Ulsan, College of Medicine, 88, Olympic-ro, 43-gil, Songpagu, Seoul, Korea
3
Research Institute for Skin Imaging, Korea University Medical Center, Guro2-dong, Guro-gu, Seoul, Korea
Keywords: Micro-endoscopy, Confocal Endoscope, Confocal Endoscopy, GRIN Lens.
Abstract: The Micro-endoscopic technology combined with optical imaging system is essential for minimally invasive
optical diagnosis and treatment in small animal disease models. Thus, the high resolution optical probe is
required to achieve high resolution imaging. However, the optical imaging system requires highly precise and
advanced technologies which are the main reasons for increasing system cost. Advancements in micro-optics
and fiber optics technology have paved way in supporting compatibility among optical components. By
providing compatibility between endoscopic system and existing conventional imaging equipment such as
macro- or micro-scope, we could achieve in not only carrying out the high quality micro-endoscopic image
procedure, but also reducing prices of the imaging system. The proposed system could be widely useful in the
field of further biological study of animal disease model.
1 INTRODUCTION
For the basic biology and preclinical study, the
experimental animal study is widely accepted for
secure confirmation the biological hypothesis.
However, the observation methods for in vivo
monitoring inside of the experimental animal are
very limited. Micro-endoscopic technology makes it
possible to visualize the inner cells and organs in
small animal with in-vivo and minimal invasively.
As the technology of the optical fiber and micro-
optics are developed, it has become essential
equipment for optical imaging diagnosis and
treatment in small animal disease models.
Recent advancement in micro-optics and fiber optics,
the miniaturized optical endoscopy probe, such as
GRIN lens assembly or fiber-bundles is utilized in
the micro-endoscope. In addition to the technology,
the high-resolution optical microscopy system is
also required for micro-endoscopes to achieve high-
quality imaging.
One of the widely used micro-endoscopy imaging
system is the confocal endoscope which enables us
high-contrast and high-resolution, real-time imaging
by taking advantage of the confocal system.
Comparing with other commercial devices from
Karl Stortz, Mauna Kea Technologis and Olympus,
the confocal endomicroscopy has higher resolution
and enables minimum invasive. At the same time,
the confocal fluorescence system allows optical
sectioning of thick tissues. However, its highly
precise micro-optical imaging system results in
increasing system cost. Besides, conventional
imaging microscopy from Leica, Zeiss and Olympus
etc. has limited working space and this is major
reason why the experimental mouse study could not
extend their applications into in vivo or live status.
In this work, the attachable micro-endoscopy system
is assembled based on commercialized confocal
fluorescence microscope by attaching the additional
optical components consist of 4f optical system,
which relays the light path from the microscope to
the endoscopy probe maintaining the optical
properties of the microscope. That expands the
capability of the microscope, not only in-vitro, but
also in-vivo imaging as well. The micro-sized triplet
GRIN(Graded-Index) lens probe is utilized to be
inserted into the body of the small animal placed on
motorized translation stage. The colon and pancreas
cells of mice are visualized by using the
implemented system for further biological study of
animal disease model.
Bae Y., Kim J. and Kim J.
Attachable Micro-endoscopy System to Conventional Microscope for Live Mouse Organ Imaging using 4f Configuration.
DOI: 10.5220/0006101001370140
In Proceedings of the 5th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2017), pages 137-140
ISBN: 978-989-758-223-3
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
137
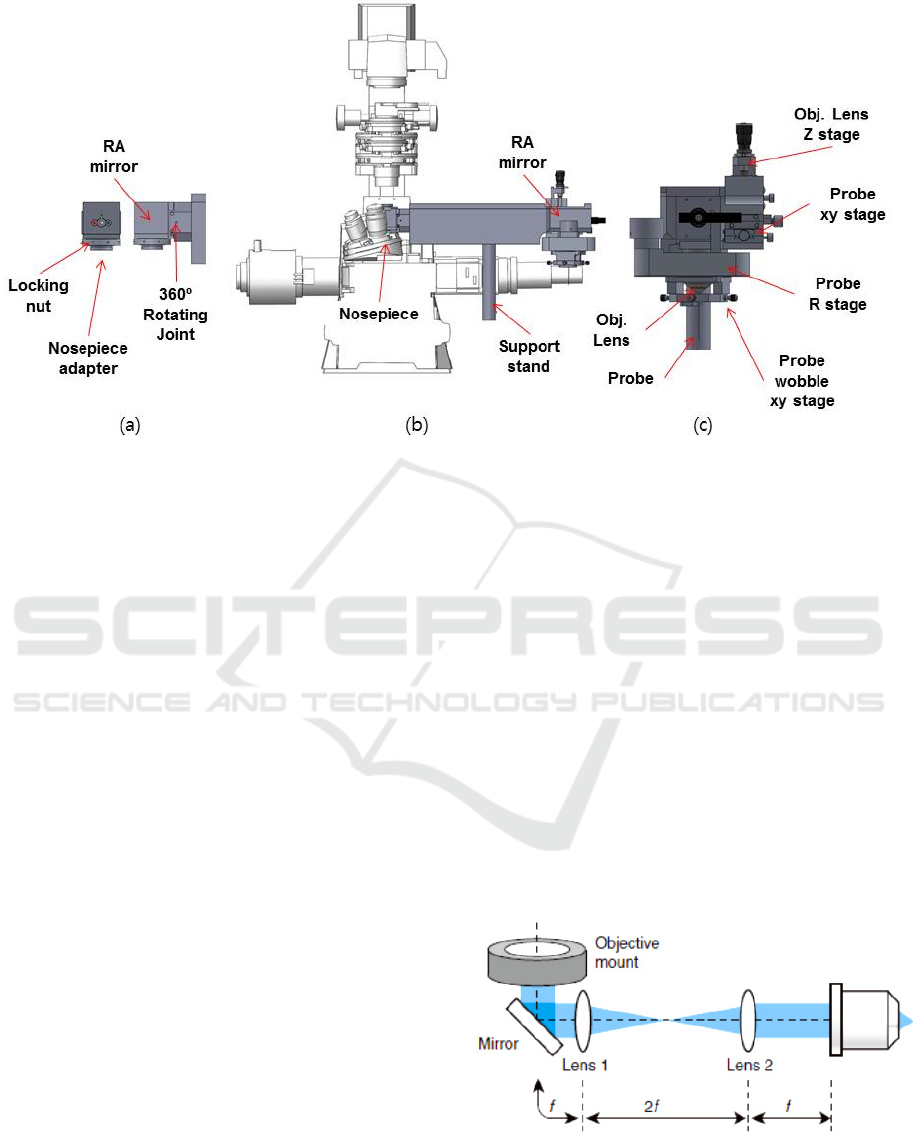
Figure 1: Illustration of the attachable micro-endoscopic system. (a) Junction part. (b) Relay optics part. (c) Endoscope
holding part.
2 DESIGN OF ATTACHABLE
MICRO-ENDOSCOPE SYSTEM
2.1 System Configuration
Fig. 1. shows the schematic illustration of the
attachable micro-endoscopy system which consists
of three parts, which are junction, relay optics and
endoscope holding part.
Junction part depicted in Fig. 1. (a) connects the
microscope to the endoscope for transmission of
lights between the microscope and the endoscope
probe. It is designed to be installed to the nosepiece
of the microscope and rotated for compatibility of
both upright and inverted microscope. The mounting
adapter in the junction part is designed to accept the
various threads of major microscope manufactures
such as Leica, Zeiss, nikon and Olympus upright /
inverted microscope etc.
The illumination light from the microscope is
reflected by the mirror in the junction part and
passes through the relay part. Then the relay part
delivers the light to the endoscope probe as shown in
Fig. 1.(b).
The delivered light is focused on the endoscope
probe by the additional objective lens inside the
endoscope holding part, shown in Fig. 1. (c).
Emission light from the sample goes back through
the attachable micro-endoscopy system then, makes
the images of the sample by the microscope. In order
to compensate the imaging wobbling during
rotational sample scanning process, the wobble stage
is built in the holding part. The distance between
endoscope and objective lens can be controlled using
Obj. Lens Z stage in Fig. 1. (c).
2.2 4f System for Light Delivery
In order to deliver the lights between the microscope
and the probe of the endoscope, the 4f optical system
is constructed in the relay optics part as shown in
Fig. 2.
Figure 2: 4f optical relay system consists of two lens and
mirror.
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
138
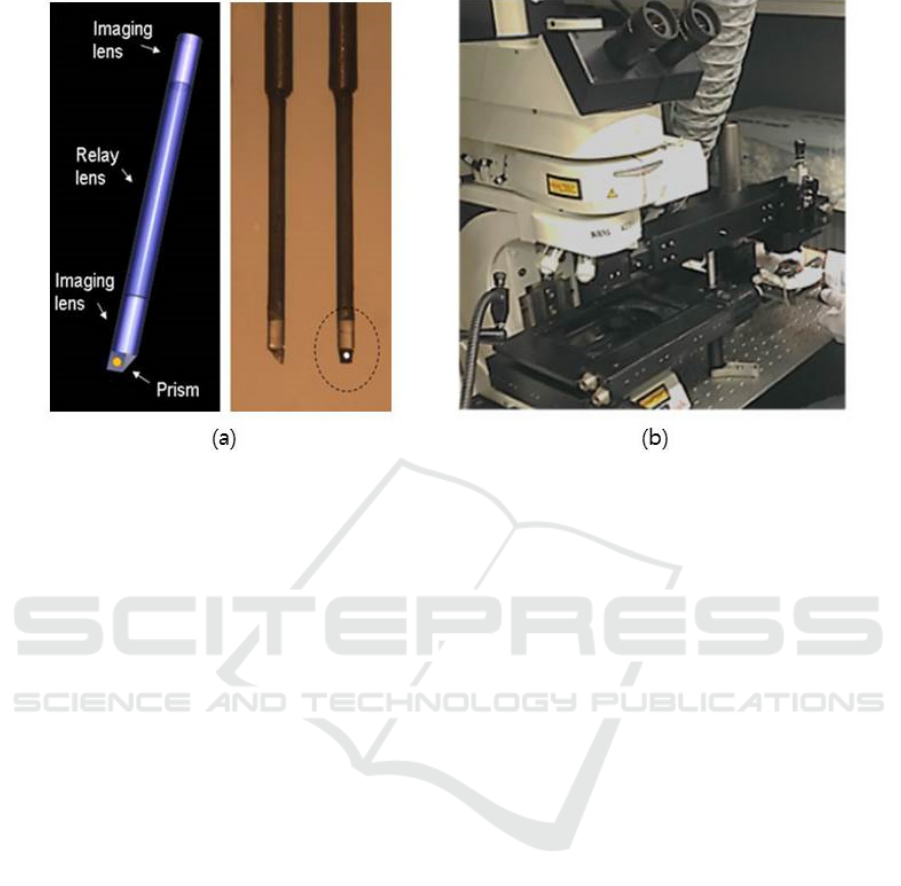
Figure 3: (a) Triplet sideview GRIN lens probe; The triplet GRIN probe consists of two imaging lens and relay lens with
micro-prism . (b) Micro-Endoscopy experimental setup combined with conventional confocal upright microscope.
It extends the beam path of the microscope to the
probe without loss of the lights power and changing
in the properties of the microscope. The system is
utilized two lenses with same focal length, f . Those
are distance 2 f each other and the objective mount
and objective lens are placed on the each sides of the
system apart from f . It is well-suited for beam
scanning microscopes such as confocal microscopes
in which the endoscopy is attached in our study. The
focal plane of the image is adjusted by translating
the axial position of the objective lens.
2.3 Endoscopy Probe and Complete
System
Triplet GRIN lens is used as the endoscopy probe in
this micro-endoscopy system, which is connected
the endoscope holding part. It should be noted that
the other types of endoscopy probe can be joined
this system such as flexible fiber-bundles. Fig. 3(a)
shows the triplet GRIN lens probe of side-view in
which angled mirror prism is adhered on the tip of
the imaging lens. The laser beam from the
microscope goes through the probe then, forms a
focal spot in front of the prism, which scans the
sample. The probe is inserted in the body of the
animal to scan the cells in the organ. The emission
light from the sample is collected by the probe and
goes back to the microscope.
There are several kinds of probe diameter from
0.35mm to 2.8mm. However, the probes of
0.35~1.0mm are mostly used for usual live mouse
imaging, since minimum invasiveness is very critical
in in vivo imaging. For the front-view probe, the
probe has no prism on the tip and this probe is more
appropriate for abdominal imaging for most organs.
Thus, front-view probe is more appropriate for
imaging most visceral organs such as liver, spleen,
kidney and so on,. For the gastrointestinal and
respiratory tracts such as colon, esophagus, trachea
and so on, side-view probe is more appropriate.
Thus, based on experimental purposes and directions,
proper probes types, diameter and length should be
chosen.
Fig. 3. (b) shows the attachable micro-endoscopy
system built on the commercialized upright confocal
microscope. The motorized translation stage is
utilized to place the sample animal and scan the
sample laterally. The micro probes are inserted into
probes hole and fixed on endoscope holder. The
complete micro-endoscopy combined with the
confocal microscopy is applied for in-vivo
fluorescence cellular imaging of internal organs in a
mouse. As a result, the experimental space has
enlarged and system costs are reduced.
3 EXPERIMENTAL RESULTS
The measured lateral and axial resolution of the
attachable micro-endoscopy system are 1μm and
10μm, respectively within 300μm of field of view,
Attachable Micro-endoscopy System to Conventional Microscope for Live Mouse Organ Imaging using 4f Configuration
139
when diameter of 1mm probe is used. That shows
sufficiently high-resolution of the system for
application of cellular imaging of mouse. The
optical penetration depth of a GRIN probes is
limited to about 100μm in most organ tissue.
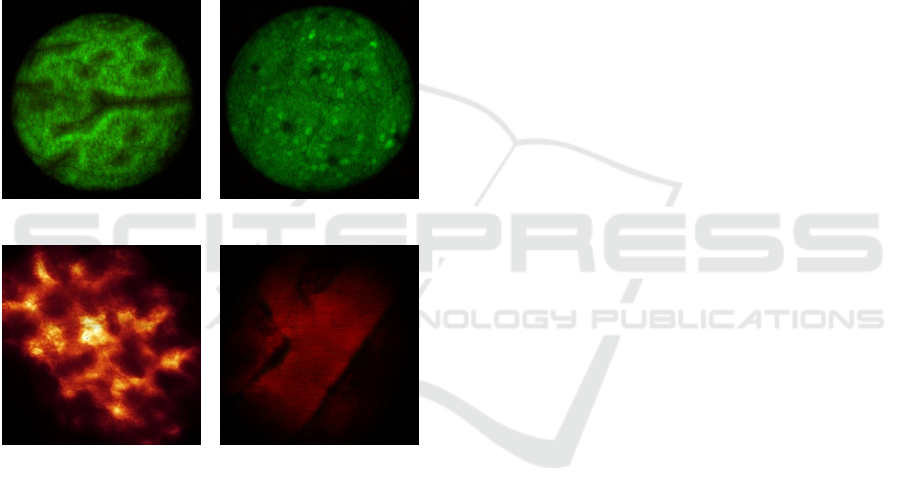
Fig. 4 shows the images of the cells in anesthetized
mouse organs taken by our system. We visualized
the mouse colon vasculature image after Acridine
Orange IV injection. Fig. 4. (a), and (b) are the
fluorescence image of mouse clone which clearly
shows the single cells in the organ. By inserting
front-view probe into live MIP+ mouse pancreas, the
pancreatic islets GFP cells are imaged. Fig. 4. (c),
and (d) are the in-vivo images of pancreatic islets
GFP cell and, blood vessel of the mouse,
respectively.
(a) (b)
(c) (d)
Figure 4: (a), (b) In-vivo images of mouse colon walls. (c),
(d) In-vivo images of pancreatic islets GFP cell, and
blood vessel of mouse, respectively.
4 CONCLUSIONS
In this paper, we present attachable micro-
endoscopy system combined with conventional
optical microscope. It features the compatibility with
the most microscope manufactures’ standards. The
developed attachable micro-endoscope system is
equipped to the conventional commercialized
confocal microscope for in-vivo cellular imaging.
The colon and pancreas cells of mice are visualized
by using the implemented system for further
biological study of animal disease model.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science
Research Program [2014R1A1A2057773,
2015K2A7A1035896] through the National
Research Foundation of Korea (NRF) funded by the
Ministry of Science, ICT & Future Planning and by
a grant (2015-641, 2015-646, 2016-7212) from the
Asan Institute for Life Science, Asan Medical
Center, Seoul, Korea.
REFERENCES
Helmchen, F. & Denk, W. Deep tissue two-photon
microscopy Nat. Methods 2, 932-940 (2005).
Jung, J. C. & Schnitzer, M. J. Multiphoton endoscopy Opt.
Lett. 28, 902-904 (2003).
JK. Kim, In vivo imaging of tracheal epithelial cells in
mice during airway regeneration, AJRCMB, 47, 864-
868 (2012)
JK. Kim, Fabrication and operation of GRIN probes for in
vivo fluorescence cellular imaging of internal organs
in small animals, Nat. Protoc. 7, 1456-1469 (2012)
Kiesslich, R., Goetz, M., Vieth, M., Galle, P.R. & Neurath,
M.F. Technology insight: confocal laser endoscopy for
in vivo diagnosis of colorectal cancer. Nat. Clin. Pract.
Oncol. 4, 480–490 (2007).
Becker, C., Fantini, M.C. & Neurath, M.F. High
resolution colonoscopy in live mice. Nat. Protoc. 1,
2900–2904 (2006).
Hsiung, P.L. et al. Detection of colonic dysplasia in vivo
using a targeted heptapeptide and confocal
microendoscopy. Nat. Med. 14, 454–458 (2008).
Dela Cruz, J.M., McMullen, J.D., Williams, R.M. &
Zipfel, W.R. Feasibility of using multiphoton excited
tissue autofluorescence for in vivo human
histopathology. Biomed. Opt. Express 1, 1320–1330
(2010).
Waldner, M.J., Wirtz, S., Neufert, C., Becker, C. &
Neurath, M.F. Confocal laser endomicroscopy and
narrow-band imaging-aided endoscopy for in vivo
imaging of colitis and colon cancer in mice. Nat.
Protoc. 6, 1471–1481 (2011).
L. Fu, Three dimensional nonlinear optical endoscopy, J.
Biomed. Opt. 12, 040501 (2007)
JK Kim, Optical fine-needle imaging biopsy of the brain,
Biomed Opt. Express, 4, 2846-2854 (2013)
PHOTOPTICS 2017 - 5th International Conference on Photonics, Optics and Laser Technology
140
