
Security Scores for Medical Devices
Johannes Sametinger
1
and Jerzy Rozenblit
2
1
Department of Business Informatics - Software Engineering, Johannes Kepler University Linz,
Altenbergerstraße 69, 4040 Linz, Austria
2
Department of Electrical and Computer Engineering, University of Arizona,
1230 E Speedway Blvd, 85718 Tucson AZ, U.S.A.
Keywords: Security, Security Score, Medical Devices, Sensitivity, Impact, Safety, Vulnerability, Security Risk.
Abstract: Medical devices are indispensable for millions of patients worldwide. They increasingly depend on software
and hardware components, and interoperate with other devices wirelessly and through the Internet. The sen-
sitive nature of health records, the increasing interoperability of medical devices, and the fact that human
well-being and life are at stake, puts medical device security at the forefront in healthcare technology. In this
paper, we contrast medical devices’ safety with their security and introduce a stratification of security scores.
We need such a grading to increase security awareness in the medical domain and as a guideline for designers
and developers who will have to act appropriately to ensure devices’ trustworthiness and as a basis for stake-
holders’ course of action when devices pose risks. We motivate and illustrate the scores by examples.
1 INTRODUCTION
Medical devices have more and more embedded soft-
ware with communication mechanisms that now
qualify them as information systems. Security is
about protecting information and information sys-
tems from unauthorized access and use. Confidential-
ity, integrity, and availability of information are core
design and operational goals. Software security is
“the idea of engineering software so that it continues
to function correctly under malicious attack”
(McGraw, 2004). In this sense, medical device secu-
rity is quite similar. Its goal is to engineer such de-
vices so that they ideally would be immune to mal-
ware implantation or if a breach occurred, they would
continue to function correctly. Medical devices com-
prise a broad range of instruments and utensils. In this
paper, we discuss only devices with hardware, soft-
ware, and some form of interoperability. For exam-
ple, most artificial joints are not “powered” by soft-
ware (yet). Thus, we can ignore them from a security
perspective. However, they are indeed critical from a
safety point of view. Researchers have demonstrated
successful hacking of medical devices on several oc-
casions. For example, Jay Radcliffe was able to send
commands to his insulin pump (raise or lower insulin
levels) and to disable it wirelessly within a distance
of up to 150 feet (Kaplan, 2011). Chunxiao et al.,
(2012) have shown security attacks and defenses for
a diabetes therapy system. FDA's safety division has
issued a warning to device makers and healthcare pro-
viders to put safeguards in place to prevent cyber-at-
tacks (FDA, 2013). We do not know about any deaths
or injuries yet, but hypothetical ramifications, e.g.,
ransomware on medical devices, are obvious. The IT
landscape can also pose a threat to medical opera-
tions. For example, when computers around the world
came to a halt after an antivirus program identified a
normal Windows file as a virus, hospitals had to post-
pone elective surgeries and stop treating non-critical
patients in emergency rooms (Fox, 2010). The fact
that there are still many medical devices based on an
old version of the Windows operating system is an-
other problem (Fu and Blum, 2013).
People increasingly manage their health and
wellness with mobile medical applications (FDA,
2015). Such apps may promote healthy living and
provide access to useful health information. Mobile
apps can extend medical devices by connecting to
them for the purpose of displaying, storing,
analyzing, or transmitting patient-specific data (FDA,
2013b). Not every mobile medical application
necessarily poses a security risk. However, as soon as
it processes or transmits sensitive information or even
controls the medical device, we have to take security
precautions. Mobile and cloud frontiers pose new
Sametinger, J. and Rozenblit, J.
Security Scores for Medical Devices.
DOI: 10.5220/0005838805330541
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 5: HEALTHINF, pages 533-541
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
533

challenges, where designers and developers of
healthcare IT must address pre-existing security
vulnerabilities and undiagnosed future threats (Kotz
et al., 2011).
Over the last years, wearable devices have be-
come popular. With sensors attached to the body, they
detect and monitor changes in body signatures of var-
ious areas. Athletes, people aware of personal fitness,
but also patients use wearable devices. For our dis-
cussion, it is not relevant whether a medical device is
wearable. Many consumer-acquired wearable devices
like fitness trackers or heart rate monitors do not qual-
ify as medical devices. They cannot have a direct neg-
ative effect on their wearers, but they may contain
sensitive information. Therefore, they are not com-
pletely out of our scope even though we concentrate
on medical devices. We propose security scores for
medical devices in order to increase security aware-
ness and, thus, to motivate stakeholders to plan coun-
termeasures accordingly. Someone's security aware-
ness is her knowledge and attitude regarding the pro-
tection of a device's information system. In the con-
text of medical devices, it is important that all stake-
holders, not just device manufacturers, display this
knowledge and attitude and, most of all, work to-
gether to explicitly know a device’s security status
and improve it when needed.
In Section 2, we briefly introduce medical devices
and contrast safety to security. In Section 3, we dis-
cuss levels of concern comprising sensitivity, impact
and exposure of medical devices. Vulnerabilities and
the suggested security scores follow in Section 4. In
Section 5, we give a discussion of the proposed
scores. A conclusion follows in Section 6.
2 MEDICAL DEVICES
Medical devices include everything from simple
wooden tongue depressors to highly sophisticated
computerized medical equipment (World Health Or-
ganization, 2003). According to the WHO, a medical
device is “an instrument, apparatus, implement, ma-
chine, contrivance, implant, in vitro reagent, or other
similar or related article” intended for use in the diag-
nosis, prevention, monitoring, treatment, etc. of a dis-
ease or other conditions (World Health Organization,
2003). The FDA uses a similar definition (FDA,
2014). Classes of medical devices are different in var-
ious countries. In the US, FDA's Center for Devices
and Radiological Health is responsible for regulating
firms, which manufacture, repackage, relabel, or im-
port medical devices. The FDA has established clas-
sifications for about 1,700 different generic types of
devices. They further group them into medical spe-
cialties, called panels. Examples for FDA’s specialty
panels include cardiovascular, dental, and orthopedic
devices (FDA, 2014).
A more general classification divides medical de-
vices into everyday use, diagnostic, therapeutic, and
life-supporting equipment (Smith, 2012). Doctors
and nurses use such equipment daily during routine
medical procedures. Examples include needles, latex
gloves, syringes and stethoscopes. The main purpose
of diagnostic equipment is to help doctors detect and
diagnose diseases. Examples include ultrasound ma-
chines, positron emission tomography (PET) scan-
ners, computer tomography (CT) scanners, and mag-
netic resonance imagery (MRI) machines. Therapeu-
tic equipment helps patients to recover and improve
their health after surgeries and other medical treat-
ments. Examples are devices such as infusion pumps
and medical lasers. Life-support equipment is helpful
in cases of physiological organ failure or major
trauma. Examples include heart-lung machines, med-
ical ventilators, and dialysis machines.
What makes medical devices stand out is not just
the fact that they may potentially threaten life. We
also need to secure our IT infrastructure. This infra-
structure comprises not only physical devices but also
personnel, security companies, emergency response
teams, etc. We typically rely upon these entities,
should IT-related problems occur. Patients and
healthcare providers are not IT experts and are very
much at the mercy of the devices’ manufacturers who
only now are beginning to take security seriously. The
goal of our suggested security scores is to fill this gap
and to make devices’ security states better accessible,
visible, and understandable to all stakeholders.
2.1 Device Safety
The FDA has assigned generic device types to the
regulatory classes I, II or III, which are based on the
level of control that is necessary to assure the safety
and effectiveness of a device. The higher a device’s
risk, the higher its class (FDA, 2014). Class I includes
devices with the lowest risk, class III those with the
highest risk. Class III devices need a pre-market ap-
proval process. Examples include implanted devices
and devices that may be necessary to sustain life like
artificial hearts or automated external defibrillators.
2.2 Device Security
Whether a medical device is active or passive is im-
portant in many respects. Passive devices do nothing
by themselves, e.g., a stethoscope or a simple artifi-
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
534

cial joint. Active devices may or may not involve
software, hardware, and interfaces, which are crucial
when considering security issues. These devices can
do some processing, receive inputs outside of the de-
vice (sensors), output values to the outer world (actu-
ators), and communicate with other devices. Medical
devices are security-critical if they do some form of
processing and communicating, typically by running
software on specialized hardware, and often, by em-
ploying a range of sensors (Sametinger et al., 2015).
The devices do not need to be re-configurable in order
to be security-relevant. All medical devices as de-
fined by the WHO or by the FDA have aspects that
are inherently safety related. However, not all of these
devices are relevant from a security point of view; re-
member the above-mentioned artificial joint. Typi-
cally, security is an issue as soon as software is in-
volved. There are, however, security-relevant instru-
ments and appliances that the WHO or the FDA do
not consider medical. Examples include smartphones
that run fitness apps handling sensitive information,
or regular PCs in a hospital for processing medical
records.
Paul et al., have proposed a security-based classi-
fication of medical devices where the primary factor
is how patients use the device (Paul et al., 2011).
Class I includes devices that are completely external
to the body. Examples include smartphones and per-
sonal computers. Naturally, devices in class I are also
a part of the medical enterprise. Class II contains de-
vices that are implanted but external to the body. Ex-
amples include infusion pumps. Finally, class III con-
tains devices that are completely implanted and are
not physically, externally accessible. Examples are
pacemakers and internal cardiac defibrillators.
2.3 Interoperability
According to a study by the West Health Institute, de-
vice interoperability with electronic medical records
(EHRs) could save the U.S. healthcare industry $30B
annually (Versel, 2013). In fact, medical devices are
increasingly communicating health information, e.g.,
insulin pumps or pacemakers may transmit logs di-
rectly to physicians or hospitals, or receive modified
settings and commands (Kramer et al., 2012). Storing
and transmitting patients’ medical information re-
quires state-of-the-art technology. Networked mobile
devices enable individuals and their physicians or
hospital personnel to better monitor and manage their
medical conditions (Kotz, 2011). If device communi-
cation is wireless or over the Internet, then transmit-
ted information is at risk of exposure. We can wire-
lessly connect devices with mobile medical applica-
tions to wearable, portable, and even embeddable sen-
sors (Kotz, 2011). They enable effortless continuous
medical monitoring. Examples of monitored values
are glucose levels in diabetic patients or the weight of
individuals seeking to lose it. In such settings, people
involved need subtle control over the collection, re-
cording, dissemination, and access to monitored data.
When patients use new sensing devices, they add a
new dimension to the confidentiality challenge. In the
near future, it is likely that we will witness the prolif-
eration of tiny sensors that detect a widening range of
compounds and report it to our mobile devices like
smartphones. We may then transmit the collected val-
ues to healthcare institutions or to public clouds, ena-
bling the powerful and cost-effective screening, diag-
nosing, monitoring, and tracking of people’s health.
Sensors and microelectronics integrated into the sole
of running shoes are one recent example. They meas-
ure the biomechanical data of the runner and evaluate
her form with real-time measurements, which they
then transmit to a smartphone and to an external
server for further evaluation. Google’s proposed
smart contact lenses to monitor diabetics provide an-
other example with a hint of how the future might
look like. Increased use of sensors may one day allow
us to monitor medical conditions and help develop in-
dividualized treatments. Clearly, the increased in-
teroperability of devices leads to increased security
risks and, hence, requires increased security measures
in order to protect these devices from attacks.
3 LEVELS OF CONCERN
The FDA has introduced the level of concern for med-
ical devices. It is a measure referring “to an estimate
of the severity of injury that a device could permit or
inflict, either directly or indirectly, on a patient or op-
erator as a result of device failures, design flaws, or
simply by virtue of employing the device for its in-
tended use” (FDA, 2005). The FDA’s severity of in-
jury distinguishes death, minor and serious injury. We
will consider device failures or design flaws in Sec-
tion 4. In addition to the impact of a device, we sug-
gest to also consider the fact whether devices store
and process sensitive information and how much a
device is exposed to its environment.
Thus, we propose a level of concern for medical
devices based on whether they process or communi-
cate sensitive information, whether they process or
communicate safety-critical information, and how ex-
posed they are to their environment. To keep things
Security Scores for Medical Devices
535
simple, we have chosen to use four levels for all cat-
egories, i.e., low, moderate, high, and very high.
3.1 Sensitivity
In a medical context, sensitive information includes
anything about a patient, e.g., medical records, and
values from sensing devices that report information
about a person’s or her device’s state, e.g., glucose
level, ID, or parameter settings of a pacemaker. We
introduce a medical device’s sensitivity to indicate
the amount of sensitive information on that device.
There are several approaches to define sensitive in-
formation in the health domain, e.g., HIV test results,
information from reproductive health clinics, and in-
formation about celebrities’ medical issues. At this
point, we emphasize that we typically do not catego-
rize information as slightly or highly sensitive. Either
it is sensitive or it is not. We propose a pragmatic ap-
proach for scoring the sensitivity and use an estimate
of the amount of sensitive information on a device,
and an estimate of how easily we can attribute this
information to a specific patient. Thus, we categorize
the information to be slightly sensitive, sensitive, or
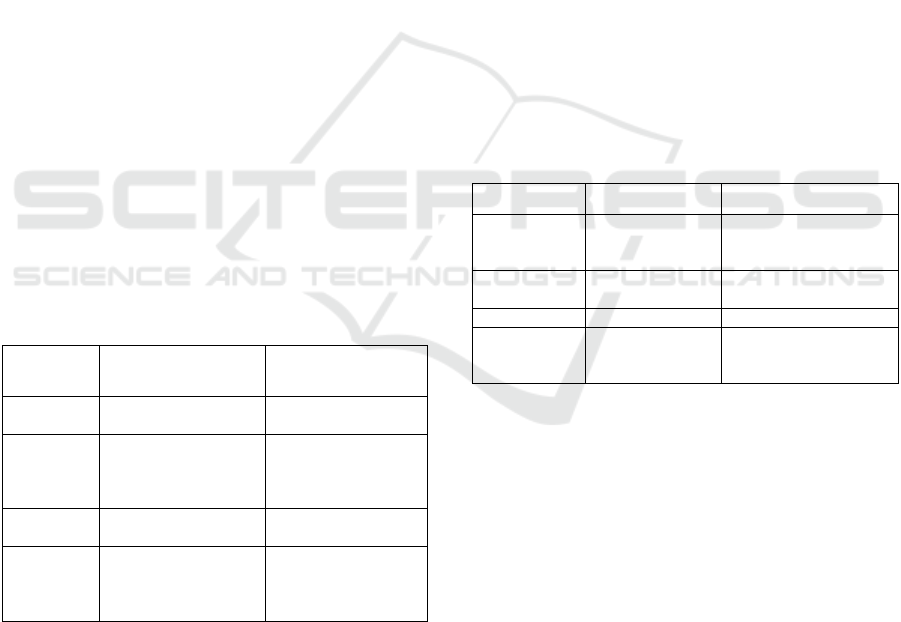
highly sensitive. Table 1 summarizes our suggested
sensitivity levels. We will later use the numbers in pa-
rentheses for calculations. We make the distinction
between high and very high based on the fact whether
sensitive information is stored of a single person or of
multiple persons. We refrain from using a finer gran-
ularity for the sake of simplicity.
Table 1: Sensitivity of medical devices.
Sensitivity Description Examples
Low (0)
No sensitive
information on device
Dental laser
Moderate
(1)
Moderately sensitive
information (sensor
values, no personal
info)
Insulin pump storing
glucose levels,
Blood glucose meter
High (2) Sensitive information
PC storing individual
health records
Very High
(3)
Highly sensitive
information (personal
information, health
information)
Server storing many
health records
3.2 Impact
Medical devices differ in the degree of impact that
they can have on a patient. Typically, devices with a
high benefit (utility) also pose a high potential harm.
For example, a cardiac pacemaker can save the life of
patients, but it can also threaten the life of a patient if
it malfunctions. A pacemaker has a direct impact on
the patient as it can directly influence the heart rate.
Other devices can have indirect impact. Determining
the indirect impact of a device is much more difficult
than determining its direct impact. For example, a
blood glucose meter has no direct impact, but the val-
ues it provides indirectly influence the insulin dose a
patient delivers to herself. This dose influences the
patient’s health. If a BGM displays wrong values,
then it may influence the amount of insulin its user
will deliver. In a worst-case scenario, this may even
result in the death of that person.
We introduce a device’s impact to indicate the in-
fluence a device has on a patient, i.e., the potential
benefit and the potential harm a device can do to a
patient, be it directly or indirectly. Table 2 summa-
rizes medical devices’ impact. Again, the coarse gran-
ularity may sometimes prohibit an easy assignment to
one of the values. For example, well-being may affect
health and the other way round. Generally, we can say
that the higher the level, the higher the impact. When
one device has a higher impact than another device,
then we assign the former to a higher level than the
latter, even though both may influence well-being or
health.
Table 2: Impact of medical devices.
Impact Description Examples
Low (0) No impact
Administrative PC in
hospital Heart rate
watch
Moderate (1)
Impact on
well-being
Drug dispensing
device, Dental laser
High (2) Impact on health Blood glucose meter
Very High
(3)
Impact on life
Pacemaker, X-ray,
Insulin pump,
Heart-lung machine
3.3 Exposure
Modern devices tend to increase interoperability. In-
teroperability refers to the mode in which devices
work with one another. Medical devices can operate
as stand-alone (low exposure) or they can interoperate
with other devices and even connect to the Internet
(high exposure). High exposure offers a big attack
surface for potential intrusions. However, it also pro-
vides flexibility and benefits. For example, a cardiol-
ogist may save a patient’s life by remotely controlling
her pacemaker in a medical emergency. An infor-
mation security exposure is “a system configuration
issue or a mistake in software that allows access to
information or capabilities that can be used by a
hacker as a stepping-stone into a system or network”
(MITRE).
A system’s attack surface is the set of ways in
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
536

which an adversary can enter the system and poten-
tially cause damage (Manadhata, 2008). Every fea-
ture of a system adds a certain amount of risk. A large
attack surface provides potential for intrusions. Nev-
ertheless, it also provides flexibility and benefits.
Table 3 summarizes medical devices’ exposure
levels. Devices connected to the Internet may have an
IP address, but they may also be accessible through
an intermediate device that connects to the Internet
and allows access to the medical device in some form.
Please note that this level is independent from any se-
curity measures that we may have taken to protect the
device. If a medical device has a high exposure, then
it is possible to control its impact and to access sensi-
tive information externally. This can be done by au-
thorized parties, e.g., medical doctors in charge, or, if
the device is not properly secured, by malicious at-
tackers. Needless to say that we need safeguards too
to prevent voluntary or involuntary harm by author-
ized parties.
Table 3: Exposure of medical devices.
Exposure Description Examples
Low (0)
Stand-alone device
without communication
features
Dental laser, Bone
growth stimulator
Moderate (1)
Device with
near-field
communication
Pacemaker
High (2)
Device with “plug-and-
play” interoperability or
distant wireless
communication
Tablets used by
physicians
Very High (3)
Device with Internet
connection
Server with
health records
3.4 Privacy and Safety Concerns
When we combine exposure with sensitivity, then we
obtain the degree of exposure of sensitive infor-
mation. If a highly exposed device stores highly sen-
sitive information, then the exposure of the sensitive
information is high. We introduce a simple formula to
calculate numerical values. By multiplying sensitivity
or impact with exposure, we get values between zero
and nine. The result is zero when either sensitivity or
exposure is zero, i.e., there neither is sensitive infor-
mation on the device nor does it interoperate with
other devices. Multiplying the values 0...3 by 0...3
yields values in the range 0,1,2,3,4,6,9. The resulting
values can be classified as low (0, 1), moderate (2, 3),
high (4, 6) and very high (9).
From a privacy perspective, we do not have to be
concerned about devices that do not store or process
sensitive information. In addition, there is no need to
be worried if the device is not exposed. From a safety
perspective, we do not have to be concerned about de-
vices without an impact on patients. Again, no need
to worry if there is no exposure of the device. From a
security perspective, devices not exposed to their en-
vironment are not an issue. At this point, we refrain
from considering situations where malware may in-
fect devices during the manufacturing process (Same-
tinger et al., 2015). Privacy is at stake if there is sen-
sitive information and we can access it from outside
the device. Safety is at stake if there is an impact on
patients and we can control this impact from outside
the device.
3.5 Examples
We discuss a blood glucose meter (BGM), a cardiac
pacemaker (CPM), an ultrasound imaging device
(USI), a magnetic resonance imagery machine (MRI),
and a diabetes logbook app (DLA) as illustrative ex-
amples. A stand-alone blood glucose meter allows its
user to read blood glucose values from the device’s
display. The device does not have any information
about the patient who uses it but may store several
historic values. An implanted cardiac pacemaker en-
ables a cardiologist to program it via wireless com-
munication. The device stores some basic information
about its wearer. An ultrasound-imaging device pro-
vides a LAN connection to store patients’ images in
an in-house database. The same is true for the mag-
netic resonance imagery machine. The diabetes log-
book app runs on a smartphone and allows its user to
log information about meals, blood glucose values
(from the BGM) as well as insulin levels and dosages.
If the app is a registered (class 1) medical device, pa-
tients’ doctors can use generated reports to make
medical decisions. The app stores backup data on the
manufacturer’s cloud infrastructure.
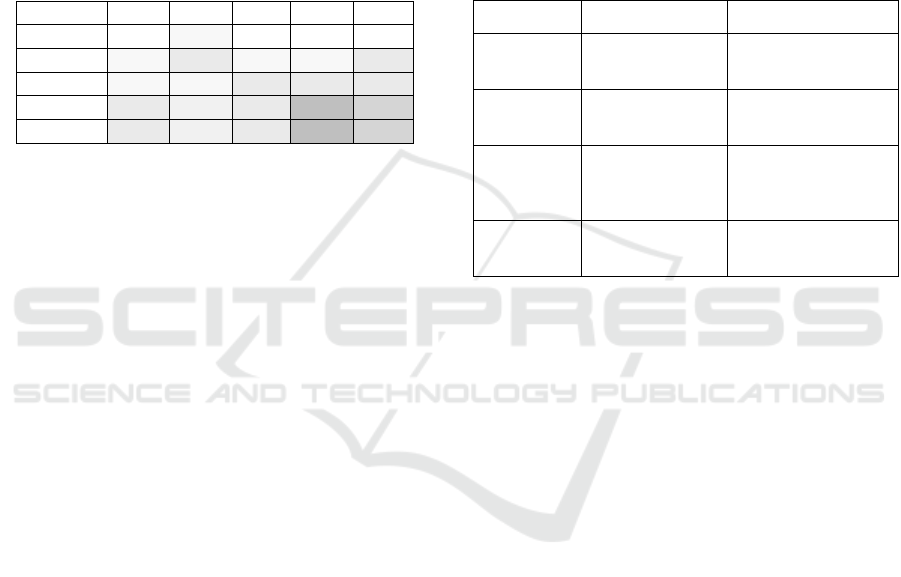
Table 4 shows all the levels of concern we have
defined for these devices. We can see at a glance
where we need security countermeasures. Please note
that the devices we have chosen as examples exist in
many different forms from various vendors. These
devices will vary in the levels of concern also. A de-
vice can store more or less sensitive information and
interoperate with its environment to a greater or lesser
extent. Therefore, it is important to define the levels
of concern for specific devices of a specific manufac-
turer, rather than for a group of devices. We have cho-
sen the numbers in Table 4 to represent a specific de-
vice category. It is interesting to see that the blood
glucose meter does not need any security precautions
even though there is an indirect impact on patients.
Missing exposure makes it impossible for attackers to
Security Scores for Medical Devices
537
manipulate the device. We have rated the impact of
the diabetes logbook app higher than the impact of the
blood glucose meter, because the logbook may con-
tain a much longer history of values that, if manipu-
lated, may have more serious consequences if used
for therapeutic decisions. We have specified different
sensitivity and impact levels for the two imaging de-
vices USI and MRI, simply to demonstrate different
level combinations. Not surprisingly, Table 4 shows
that our MRI needs more security precautions than
our BGM.
Table 4: Levels of concern for sample medical devices.
Device Sn Im Ex PLC SLC
BGM 0 1 0 0 0
CPM 1 3 1 1 3
USI 1 1 3 3 3
MRI 3 2 3 9 6
DLA 3 2 3 9 6
Sn … Sensitivity, Im … Impact, Ex … Exposure
PLC … Privacy level of concern, SLC … Safety level of concern
BGM … Blood glucose meter, CPM … Cardiac pacemaker
USI … Ultrasound imaging device, MRI … Magnetic resonance imagery
DLA … Diabetes logbook app
4 SECURITY SCORES
Regardless of a device’s level of concern, i.e., its sen-
sitivity, impact, and exposure, it can be secure or in-
secure depending on whether there are vulnerabilities
and on whether somebody knows these. “100% se-
cure” devices are as unlikely as zero-fault software.
We will have to find a way to manage insecure de-
vices. Devices with high exposure of sensitive infor-
mation pose a higher privacy risk, but if we have
taken proper security countermeasures, then the pri-
vacy threat may still be low. The same holds for de-
vices with a high exposure of impact and a higher
safety risk. Whether a device is actually at risk de-
pends on whether there are security vulnerabilities
and potential exploits. Thus, we have to define the
current vulnerability level and then calculate the pri-
vacy and safety scores depending on a device’s sen-
sitivity and impact, respectively.
4.1 Vulnerability
We have stated in the introduction that secure devices
have to continue to function correctly even if under a
malicious attack. Vulnerabilities are errors in devices,
typically in software, which we can directly use to
gain unauthorized access to the device. They pose a
threat to the device itself, to the information it con-
tains, to other devices it communicates with, and to
its environment. Today, most stakeholders are con-
cerned about the safety of medical devices. They pay
less attention to the devices’ vulnerabilities. These
can be quite volatile. When we detect vulnerabilities,
our rating of the device’s threat may increase rapidly.
It is important for everyone involved to have a clear
picture of the current security status and to make rea-
soned decisions about necessary steps in order to de-
crease the threat, if needed. We propose vulnerability
levels as described in Table 5.
Table 5: Vulnerability of medical devices.
Security Description Examples
Low (0)
Neither
vulnerabilities nor
malware on device
New device
Device with upgraded
software version
Moderate (1)
Vulnerabilities
on device,
no exploits yet
Weakness in protocol
Potential buffer
overflow
High (2)
Vulnerabilities
on device with
known exploits
Protocol weakness or
buffer overflow can be
used for unauthorized
access
Very High (3) Malware on device
Hardware Trojan or
software backdoor on
device
At this point, we clearly have to distinguish be-
tween two different perspectives, i.e., the attacker’s
and the risk analyst’s perspective. If a potential at-
tacker detects vulnerabilities, then the actual threat,
but not necessarily the analyst's assessment of it, will
increase. Thus, a device’s vulnerability level repre-
sents a person’s knowledge about this device’s vul-
nerabilities. Not knowing any vulnerability does not
necessarily mean that none exists. A device’s vulner-
ability provides a dynamic property, as found vulner-
abilities and exploits increase the risk, and later
patches and updates reduce the risk.
Both software and hardware take time to mature.
Therefore, a five year old product may potentially be
safer than a new product out in the market. However,
we argue that it is reasonable to assume that a new
device or software upgrade is low on the vulnerability
scale, cf. Table 5. At that time, vulnerabilities are not
yet known and, for software upgrades, known vulner-
abilities have usually been fixed.
4.2 Privacy and Safety Scores
A medical device is at risk when it is vulnerable and
stores sensitive information. It also poses a risk when
it is vulnerable and it has an impact on a patient. Pri-
vacy concerns exist when personally identifiable in-
formation or other sensitive information is processed
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
538
or stored. Insufficient access control or inappropriate
sharing are often the cause of privacy issues. Privacy
is about the ability to conceal information about a pa-
tient. Disclosure of sensitive information may result
in negative consequences. For example, an employer
may not be willing to employ people with HIV. Sen-
sitive information may also constitute a threat, be-
cause wrong values may later induce therapeutically
wrong decisions by doctors or devices. We calculate
privacy and safety scores again by using multiplica-
tion. Sensitivity multiplied by the vulnerability yields
the privacy score; impact multiplied by vulnerability
yields the safety score.
The levels of concern provide general information
about how important security precautions are for a de-
vice. Vulnerability levels and security scores, i.e., pri-
vacy and safety score, indicate a device’s current se-
curity status. The higher it is, the more we have to
expect security breaches.
4.3 Examples
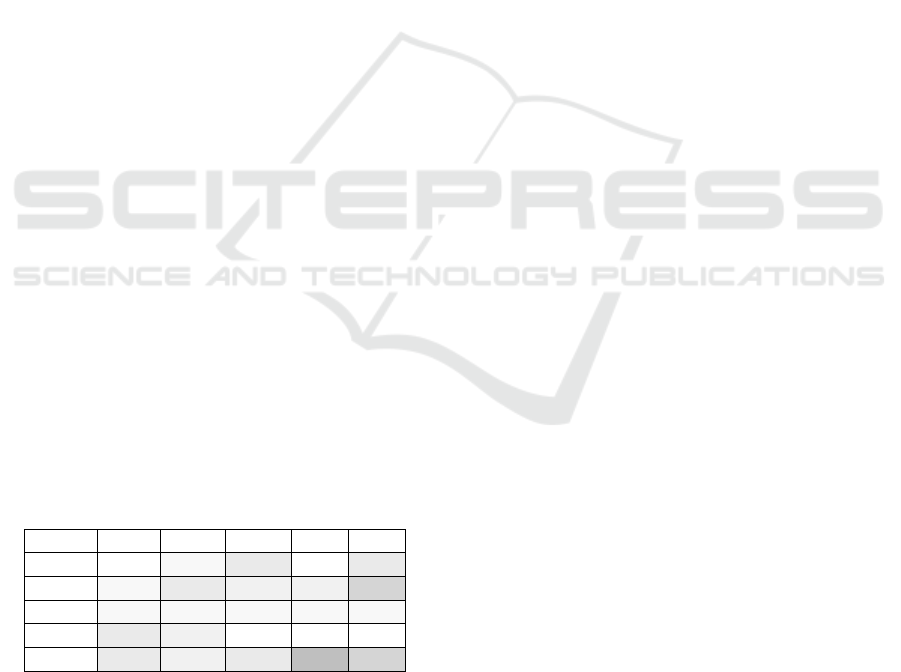
In Table 6, we present different vulnerability levels
for the devices introduced in the previous section. If
we assume that there are no known vulnerabilities,
then we set the appropriate level to zero. This is the
case, for example, when a new device comes to mar-
ket. There is no risk yet at that time. Later, when we
know about vulnerabilities, risk increases. In Table 6,
we show devices as defined above with various vul-
nerability levels and the resulting privacy and safety
scores. A quick glance at the table reveals that privacy
and safety are at risk in the logbook app, and that the
cardiac pacemaker’s safety is at risk. We will discuss
consequences from that information below. Please
note that we use values for sample devices in Table 6.
These devices may have different values than speci-
fied in our table. The values depend on the specific
implementation and equipment by the manufacturers.
Table 6: Privacy and safety scores for sample devices.
Device Sn Im Vu Priv Saf
BGM 0 1 3 0 3
CPM 1 3 2 2 6
USI 1 1 1 1 1
MRI 3 2 0 0 0
DLA 3 2 3 9 6
Sn … Sensitivity, Im … Impact, Vu … Vulnerability
Priv … Privacy Score, Saf … Safety Score
BGM … Blood glucose meter, CPM … Cardiac pacemaker
USI … Ultrasound imaging device, MRI … Magnetic resonance imagery
DLA … Diabetes logbook app
We have to mention at this point that knowledge
about the existence or non-existence of vulnerabilities
may differ among different persons. It is always pos-
sible that only malicious attackers are aware of vul-
nerabilities. In this case, we may assume a low score
even though the danger of an attack is indeed high.
The malicious attackers who know about the vulner-
ability are able to determine the appropriate score.
5 DISCUSSION
We imagine having levels of concern as well as pri-
vacy and safety scores defined for any medical de-
vice. Levels of concern include sensitivity, impact,
and exposure. Manufacturer can define them for the
approval process. The vulnerability level as well as
privacy and safety scores of a device are dynamic
properties; we have to maintain them in order to de-
scribe the current risk associated with using a device.
It should be mandatory that they be maintained and
publicly available. Thus, doctors and patients would
be able to check out both the levels of concern and
security scores of a specific device. As manufacturers
may be hesitant to admit increased vulnerability, we
imagine a neutral third-party organization to be in
charge, e.g., the MITRE Corporation that operates
CVE, a dictionary of publicly known information se-
curity vulnerabilities and exposures. We may deduct
a device’s vulnerability level from the number and se-
verity of its CVEs.
Risk management includes risk framing, risk as-
sessment, risk response and risk monitoring (Ross,
2011). Our suggested security scores contribute to the
assessment and to the monitoring of risks of medical
devices. The number of entry points that an attacker
may use to access the device defines the attack sur-
face. The exposure we have suggested does not dif-
ferentiate between devices with big or small attack
surfaces. Minimizing the attack surface can reduce
the risk of attacks, but it does not change the general
exposure of a device. Proper authentication and au-
thorization also makes a huge difference in securing
a device. If a device’s manufacturer has chosen to im-
plement adequate security measures like authentica-
tion or encryption, then the level of concern will be
unchanged. However, these measures will affect pri-
vacy and safety scores, because proper security mech-
anisms will have an effect on the emergence of vul-
nerabilities.
We definitely need mandatory security analyses
for devices that may threaten human life. Our pro-
posed scores and levels of concern provide a black-
box view to medical devices. If manufacturers ana-
Security Scores for Medical Devices
539

lyze security properly, it will later have a positive ef-
fect on the number of vulnerabilities that we will get
to know. We will see positive effects in low values
for our privacy and safety scores. Threat analysis is
another activity that we need to evaluate security risks
posed to a device. Such analysis is important in order
to plan for countermeasures. A device manufacturer
should include such activities into the development of
the devices. The results will not be publicly available,
as it would provide valuable information to attackers.
We do not use such information to characterize med-
ical devices, but they will have a positive effect to
their security scores.
We have presented a numerical system that still
represents work in progress. As a next step, we plan
to perform a proof of concept. The fundamental prin-
ciple of design science research is that we acquire
knowledge of a design problem and its solution in the
creation and application of design artifacts (Hevner,
2007). The research outcome not only includes the
design artifact itself but also a clearly defined contri-
bution to scientific knowledge. Another next step is
to define rules of action, such that stakeholders have
defined process models as guidance for further action.
Further action can be manifold and depends on the
device. For example, if we have a safety score of nine
in a pacemaker, then one option is to remove the pace-
maker from the patient and replace it with another
model. A less expensive and less time-consuming al-
ternative is to provide future medical devices with an
option to cut off communication features. Whatever
the response to an increased risk of a device might be,
we should have predefined courses of action in order
to act quickly. The security scores described in this
paper are a first step in this direction.
6 CONCLUSIONS
Medical devices increasingly use wireless communi-
cation and Internet connections. Additionally, we see
an increased use of mobile medical applications in
connection with a plethora of medical sensors still to
come. We have introduced security scores in an effort
to increase the security awareness of all involved par-
ties and to provide a knowledge base that makes it
possible to make sound decisions in different security
situations. Sensitivity, impact, and exposure are static
properties of devices. They reflect whether a device
handles sensitive or safety-critical information and
how exposed it is to its environment. Vulnerability
and risk are dynamic. If they increase, we have to take
appropriate countermeasures, or the doors will stand
wide open for the misuse of sensitive medical data
and for malware and attacks that put human life in
danger.
We have not provided any information on how to
implement effective defense mechanisms. It is the
manufacturer’s task to pay due attention to the devel-
opment of secure devices. Our suggested scores can
provide information about how concerned we have to
be in general about security precautions of specific
devices and about security and safety risks at specific
points of time. The consequences may be manifold.
Manufacturers may fix problems or patients and hos-
pitals may decide to refrain from using these devices.
REFERENCES
Chunxiao, L., Raghunathan, A., Jha, N. K., 2011. Hijacking
an insulin pump: Security attacks and defenses for a di-
abetes therapy system. 13th IEEE International Confer-
ence on e-Health Networking Applications and Services
(Healthcom), pp 150-156. https://ieeexplore.ieee.org/
xpl/articleDetails.jsp?arnumber=6026732
FDA, 2005. Guidance for the Content of Premarket Sub-
missions for Software Contained in Medical Devices,
May 11, 2005.
http://www.fda.gov/MedicalDevices/DeviceRegulatio
nandGuidance/GuidanceDocuments/ucm089543.htm
FDA 2013. FDA Safety Communication: Cybersecurity for
Medical Devices and Hospital Networks. June.
http://www.fda.gov/MedicalDevices/Safety/Alertsand
Notices/ucm356423.htm
FDA, 2013b. Mobile Medical Applications – Guidance for
Industry and Food and Drug Administration Staff. Sept.
2013.
http://www.fda.gov/downloads/MedicalDevices/Devi
ceRegulationandGuidance/GuidanceDocuments/UCM
263366.pdf
FDA, 2014. Medical Devices – Classify Your Medical De-
vice. http://www.fda.gov/MedicalDevices/DeviceRegu
lationandGuidance/Overview/ClassifyYourDevice/de
fault.htm
FDA, 2015. Mobile Medical Applications – Guidance for
Industry and Food and Drug Administration Staff, Feb.
09. http://www.fda.gov/downloads/MedicalDevices/.../
UCM263366.pdf.
Fox News, 2010. Antivirus Program Goes Berserk, Freezes
PCs. April 22. http://www.foxnews.com/tech/2010/
04/22/antivirus-program-goes-berserk-freezes-pcs/
Hevner, A. R., 2007. A Three Cycle View of Design Sci-
ence Research, Scandinavian Journal of Information
Systems, Vol. 19: Issue 2, Article 4.
http://aisel.aisnet.org/sjis/vol19/iss2/4
Fu K. and Blum J., 2013. Controlling for cybersecurity risks
of medical device software, Communications of the
ACM, vol. 56, no. 10, p. 35.
Kaplan D., 2011. Black Hat: Insulin pumps can be hacked.
SC Magazine, August 04. http://www.scmagazine.com/
black-hat-insulin-pumps-can-be-hacked/article/209106
SmartMedDev 2016 - Special Session on Smart Medical Devices - From Lab to Clinical Practice
540

Kotz, D., Fu, K., Gunter, C., and Rubin, A., 2015. Security
for mobile and cloud frontiers in healthcare, Communi-
cations of the ACM, vol. 58, no. 8, pp. 21–23.
Kramer, D. B., Baker, M., Ransford, B., Molina-Markham,
A., Stewart, Q., Fu, K., Reynolds, M. R., 2012. Security
and Privacy Qualities of Medical Devices: An Analysis
of FDA Postmarket Surveillance. http://www.plosone.
org/article/info:doi/10.1371/journal.pone.0040200
McGraw, G., 2004. Software Security, IEEE Security &
Privacy, vol. 2, no. 2, pp. 80-83, March-April.
doi:10.1109/MSECP.2004.1281254
Kotz, D., 2011. A threat taxonomy for mHealth privacy,
Workshop on Networked Healthcare Technology
(NetHealth), January. http://ieeexplore.ieee.org/xpl/ar-
ticleDetails.jsp?arnumber=5716518
Paul, N., Kohno, T., Klonoff, D. C., 2011. A Review of the
Security of Insulin Pump Infusion Systems, Journal of
Diabetes Science and Technology, vol. 5, Issue 6.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3262
727
Manadhata, P., 2008. An Attack Surface Metric, CMU-CS-
08-152. http://reports-archive.adm.cs.cmu.edu/anon/
2008/CMU-CS-08-152.pdf
MITRE. The MITRE Corporation: Common Vulnerabili-
ties and Exposures – The Standard for Information Se-
curity Vulnerability Names. https://cve.mitre.org
Ross, R. S., 2012. Guide for Conducting Risk Assessments,
NIST Special Publication 800-30 Revision 1.
http://csrc.nist.gov/publications/nistpubs/800-30-rev1/
sp800_30_r1.pdf
Sametinger, J., Rozenblit, J., Lysecky, R., and Ott, P., 2015.
Security Challenges for Medical Devices, Communica-
tions of the ACM, vol. 58, no. 4, pp. 74–82.
Smith, E., 2012. Types of Medical Equipment. HIVE
Health Media. January 22. http://www.hivehealthme
dia.com/types-medical-equipment/
Versel, N., 2013. West: Device interoperability with EHRs
could save $30B annually. Mobihealthnews.
http://mobihealthnews.com/21120/west-device-interop
erability-with-ehrs-could-save-30b-annually/
World Health Organization, 2003. Medical device regula-
tions: global overview and guiding principles, ISBN
92-4-154618-2. http://whqlibdoc.who.int/
publications/2003/9241546182.pdf
Security Scores for Medical Devices
541
