
Multiple Source Phototherapy in Breast Cancer: A Viability Study
A. Lopes
1,2
, A. Gabriel
1,2
, J. Machado
1,2
, P. Ribeiro
1,3
, R. Gomes
4,5
, Jo
˜
ao M. P. Coelho
4,5
,
C. O. Silva
6
, C. P. Reis
5,6
, J. P. Santos
1,2
and P. Vieira
1,2
1
Department of Physics, Faculdade de Ci
ˆ
encias e Tecnologia da Universidade Nova de Lisboa,
R. Quinta da Torre, Monte da Caparica, Portugal
2
LIBPhys, Faculdade de Ci
ˆ
encias e Tecnologia da Universidade Nova de Lisboa, Monte da Caparica, Portugal
3
CEFITEC, Faculdade de Ci
ˆ
encias e Tecnologia da Universidade Nova de Lisboa, Monte da Caparica, Portugal
4
Laborat
´
orio de
´
Optica, Lasers e Sistemas, Faculdade de Ci
ˆ
encias da Universidade de Lisboa, Lisboa, Portugal
5
Instituto de Biof
´
ısica e Engenharia Biom
´
edica, Faculdade de Ci
ˆ
encias da Universidade de Lisboa, Lisboa, Portugal
6
CBiOS - Centre for Research in Biosciences & Health Technologies, Lus
´
ofona University, Lisboa, Portugal
Keywords:
Near Infrared, Spectroscopy, GAMOS, Monte Carlo, Phototerapy, Breast Cancer.
Abstract:
Radiation therapy is one of many common treatments applied to breast cancer. Most usual radiation sources ap-
plied are ionizing radiation, such as γ-rays and X-rays, and non-ionizing radiation such as ultraviolet radiation.
The possibility of using near infrared light to photoactivate a drug inside an 8 cm diameter biological object is
discussed in this work via Monte Carlo simulations. Two simulation setups performed in the Geant4/GAMOS
framework are presented in order to study the viability of photoactivating a drug by using several near infrared
light sources. The overall objective of this technique is to minimize energy concentrated at objects surface
and maximize it in a predefined region of interest. Results show an increase energy absorption in the desired
region of interest inside a 8 cm object, when a higher absorption particle is present. With the use of multiple
sources it is possible to photoactivate the drug while causing minimal damage to the surface of the radiated
object.
1 INTRODUCTION
Radiation therapy, or radiotherapy, is one of the stan-
dard treatments for patients with breast cancer. Con-
ventional ionizing radiotherapy is performed using X-
rays and γ-rays combined with chemotherapy. This
method is usually employed after surgery to improve
cancer treatment (Sarkar et al., 2013). On the other
hand non-ionizing ultraviolet (UV) radiation, which
utilizes phototherapy techniques, is employed to treat
skin cancer diseases which can develop from ionizing
radiation therapies(Costa et al., 2014). There are sev-
eral advantages and disadvantages to either of these
types of ionizing and non-ionizing radiations. In the
first case radiation will penetrate the biological tis-
sue but it is known to cause serious side effects that
one must take into account. In the latter case effective
low light penetration into subcutaneous tissue is the
biggest disadvantage (Sarkar et al., 2013).
To study the possibility of using near infrared
(NIR) radiation is one of the aims of this work. NIR
light is a non-ionizing radiation that produce even
less undesired side effects and has greater effective
penetration than UV radiation. NIR light sources
would be applied in treatment of breast tissue and
other melanoma beyond the subcutaneous surface by
photoactivating gold nanoparticles with drug carry-
ing capabilities and biocompatible coatings. The use
of multiple radiation sources to minimize skin le-
sions and optimize energy in a specific region is the
main idea behind this work and was also discussed
in (Gabriel et al., 2015). By adding multiple sources
our intention is to optimize the energy ratio between
biological tissue surface and a predefined region of
interest. Monte Carlo simulations were carried on
to study this possibility as they are the reference in
the realm of simulations of light interactions with bi-
ological tissues (Zhu and Liu, 2013). To perform this
simulation it is used the Geant4/GAMOS framework
which has already been validated by other authors as
shown in (Glaser et al., 2013; Morhard et al., 2014).
Geant4 is a powerful simulation tool that was devel-
oped for nuclear and particle physics experiments.
GAMOS framework offers the necessary extension of
Lopes, A., Gabriel, A., Machado, J., Ribeiro, P., Gomes, R., Coelho, J., Silva, C., Reis, C., Santos, J. and Vieira, P.
Multiple Source Phototherapy in Breast Cancer: A Viability Study.
DOI: 10.5220/0005794902470250
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 247-250
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
247

Geant4 to perform Monte Carlo simulations for Med-
ical Physics applications. Its tissue optics plug-in was
also used because it offers the possibility of simulat-
ing photons interacting with biological tissue and in
the NIR range of the spectrum.
2 MATERIALS AND METHODS
2.1 Geometry and Optical Properties
The input parameters as well as the scattering theory
used in GAMOS were firstly defined in order to begin
the simulation. The use of literature values to perform
this study was our first approach. However several pa-
rameters for the same variables in different references
were found (van Veen et al., 2004; Jacques, 2013)
which produced distinct simulations results. The out-
put results from these parameters were not consis-
tent with other groups’ experimental results (Gibson
et al., 2005) because photons were not penetrating
deep enough into the tissue. To overcome these dif-
ficulties, we conducted an experiment in which the
optical properties of a piece of pig lard were mea-
sured, and determined the best simulation parame-
ters fitted to experimental results. These results will
be published elsewhere and were based on (Gaigalas
et al., 2009). In the present work it is assumed the
simulated photons’ interaction with the pig lard pro-
duce similar results as photons’ interaction in breast
tissue. The Mie Henyey-Greenstein scattering the-
ory model was chosen because it has been proven by
other groups to be the best to perform these kind of
simulations (Jacques, 2013). To determine the Mie
and anisotropy scattering coefficients we use a MAT-
LAB script based on the work described in (Bohren
and Huffman, 2007) and developed by Scott Prahl and
Christian Maetzler in (Jacques and Maetzler, 2002).
This software calculates both coefficients given aver-
age sphere dimensions, fractional volume and refrac-
tive indexes of tissue. The selected simulations in-
put values were the ones which matched our pig lard
experimental results and were chosen from a wide
range of values taken from several referenced articles
(Jacques, 2013; Wang et al., 2005; Jacques, 1996).
The refractive index of the simulated object was taken
from (Bashkatov et al., 2005). Two different setups
were simulated and are described in the next para-
graphs.
2.1.1 Setup #1
To minimize geometry dependences, we performed a
simulation in a homogeneous cylinder with 8 cm di-
Figure 1: System geometry for setup #2.
ameter and 4 cm height as a first approach for mod-
elling a section of the tumour site. Future work will
comprise the study of the geometry contribution to the
simulation results, namely the dependence on the an-
gle between the beam axis and the solid surface and
when one considers different solid geometries.
2.1.2 Setup #2
A sphere was included in the centre of the volume of
setup #1 with 1 cm diameter and higher absorption. It
intents to mimic the optical properties of the photoac-
tivated drug that its supposed to be aggregated around
the tumour. Gold nanoparticles to be activated in the
NIR range were developed by other members of this
project. They also measured their optical properties
so the spheres absorption coefficient was estimated
based on their studies. These studies will be pub-
lished elsewhere. A picture of this setup is displayed
in Fig.1.
Both simulation setups consider 16 sources with
a wavelength of 810 nm each aimed to the centre of
the cylinder, equally spaced out around the object and
equidistant from its lateral surface.
Input values of the GAMOS simulations are
shown below:
• Wavelength: 810 nm
• Refractive Index: 1.44
• Mie scattering coefficient: 221.69 cm
−1
• Scattering anisotropy: 0.97
• Cylinders absorption coefficient: 0.01 cm
−1
• Spheres absorption coefficient: 1.00 cm
−1
For each setup 2 million events were generated.
Each source has 1 ps pulse with Gaussian distribution
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
248
in wavelength and position of σ
λ
= 3 nm and σ
p
= 0.5
mm, respectively.
3 RESULTS
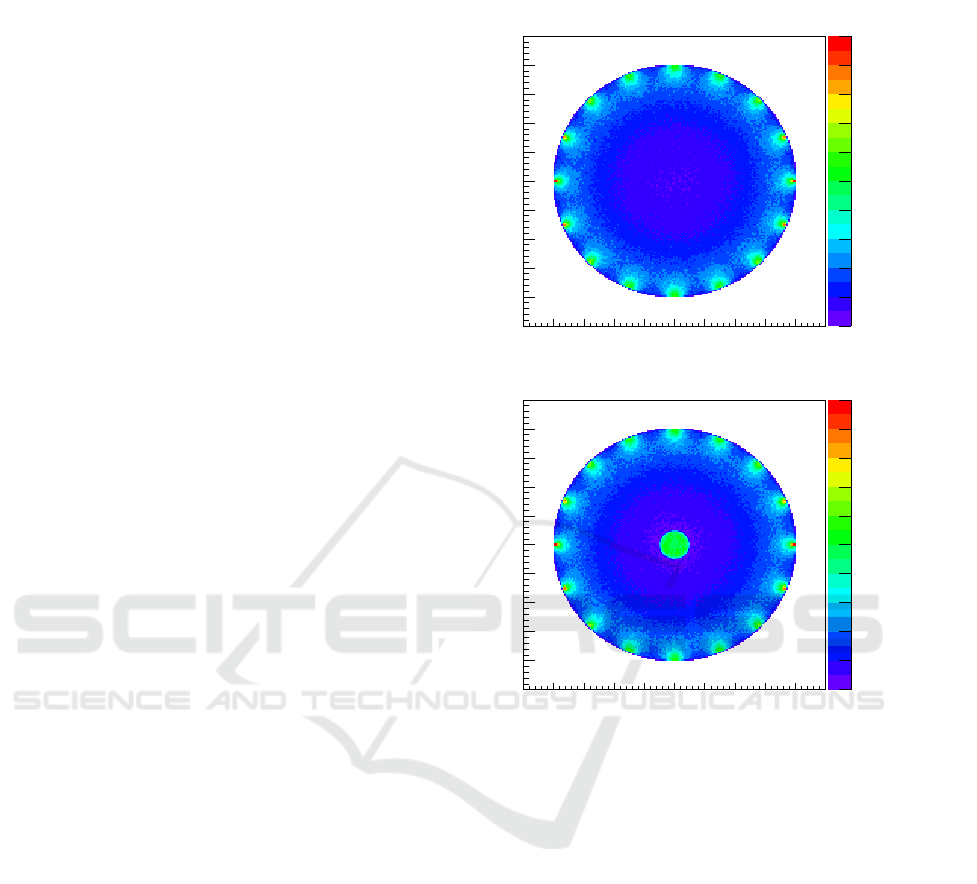
The simulation results for setup # 1 are presented
in Fig.2 which shows a top-view of the absorption
interactions of photons with the tissue inside the
cylinder. The X-Y plane is measured in mm while
the Z-axis represents the number of counts in each
X-Y bin and is integrated in height. As expected from
an homogeneous tissue the results show equivalent
number of interactions when considering the same
radius. The exception lies at the edge of the object
where photon beams enter the tissue, where one can
see a higher number of counts. This behaviour is
expected because of the higher density of photons
present in where the photon beams are aimed.
The simulation results for setup # 2 are also
displayed in Fig.2. A top view of the absorption
interactions inside the cylinder setup is presented.
Besides demonstrating the same radius dependency
on absorbed photons and the same higher density on
the number of counts where the photon beams are
aimed, this result also show a higher density on the
number of counts inside the object where the higher
absorption sphere lies.
Energy densities present in the region of interest
were computed with and without the sphere, consid-
ering the number of photons that are absorbed in the
predefined region of interest. When one does not
consider the absorber sphere in the centre of the ob-
ject the energy density is 1.2 × 10
−27
J/cm
3
. When
the absorber sphere is present the energy density is
40 × 10
−27
J/cm
3
.
4 DISCUSSION
Scattering and absorption simulation studies of pho-
tons interaction with biological tissues were studied
in (Gabriel et al., 2015). Absorption interactions with
the tissue are studied in this work. There are two rea-
sons for presenting only absorption studies. Firstly,
when considering a same volume, there is more than 1
absorption interaction per each 10000 scattering inter-
actions in average. This can be showed with the ratio
between the scattering and absorption coefficients. If
we proceeded with scattering and absorption interac-
tion plots the results would be masked as fluctuations.
# counts
0
100
200
300
400
500
600
700
800
900
1000
X (mm)
-50 -40 -30 -20 -10 0 10 20 30 40 50
Y (mm)
-50
-40
-30
-20
-10
0
10
20
30
40
50
# counts
0
100
200
300
400
500
600
700
800
900
1000
X (mm)
-50 -40 -30 -20 -10 0 10 20 30 40 50
Y (mm)
-50
-40
-30
-20
-10
0
10
20
30
40
50
Figure 2: Absorption interactions inside the object of setup
#1 on top and setup # 2 below.
Also only when photons are absorbed the drug is pho-
toactivated by the deposited energy. The most impor-
tant remark on the results of setup #2 when compared
to setup #1: there is a greater number of absorption
interactions in the centre of the object due to higher
absorption of the drug supposedly aggregated to the
tumour.
The results present in this paper also give a no-
tion of what kind of energy ratio is expected between
the location of the beam entrance and the region of
interest where the drug is located. Enhancement of
this ratio allow prevention of skin lesions when try-
ing to photoactivate the drug. Another possible way
of increasing this ratio is using multiple sources offset
in time in order to create constructive interferences in
the interest region within the tissue.
Since we are considering the same generated
events between the two setups, one can compare the
energy density among the two setups, and it is approx-
imately 30 times higher. This is also an indicator of
Multiple Source Phototherapy in Breast Cancer: A Viability Study
249

the viability of this radiation technique.
5 CONCLUSIONS
We have presented a study about the use of NIR light
to photoactivate a drug which aggregates around the
tumour site inside an object with 8 cm. We have
shown by using multiple sources for irradiating an ho-
mogeneous tissue absorption interactions behave sim-
ilarly on equal radius distances, while minimizing the
energy absorption at its surface. When higher absorp-
tion drug particles are simulated inside the object re-
sults show they can be photoactivated thus enabling
treatment in the tumour area, while minimizing the
damage to the surrounding healthy tissues.
In future work it will be important to make the
model more realistic by including skin and vasculari-
sation. It will be also important to optimise the source
distribution and modulation in order to maximize the
power delivery in the region of interest.
ACKNOWLEDGMENTS
This work was partially supported by national
funding by the Portuguese FCT - Fundac¸
˜
ao para
a Ci
ˆ
encia e Tecnologia through the projects
PTDC/BBB-BMD/0611/2012, UID/BIO/00645/2013
and PD/BD/105920/2014.
REFERENCES
Bashkatov, A. N., Genina, E. A., Kochubey, V. I., and
Tuchin, V. V. (2005). Optical properties of human
skin, subcutaneous and mucous tissues in the wave-
length range from 400 to 2000nm. Journal of Physics
D: Applied Physics, 38(15):2543.
Bohren, C. F. and Huffman, D. R. (2007). Appendix A:
Homogeneous Sphere, pages 477–482. Wiley-VCH
Verlag GmbH.
Costa, M. M., Silva, S., and et al. (2014). Phototherapy 660
nm for the prevention of radiodermatitis in breast can-
cer patients receiving radiation therapy: study proto-
col for a randomized controlled trial. BioMed Central,
15.
Gabriel, A., Machado, J., Gomes, R., Coelho, J., Silva, C.,
Reis, C., Santos, J., and Vieira, P. (2015). Concen-
trated photoactivation: focusing light through scat-
tering. In World Congress on Medical Physics and
Biomedical Engineering, June 7-12, 2015, Toronto,
Canada, volume 51, pages 1727–1730, Toronto,
Canada.
Gaigalas, A. K., He, H.-J., and Wang, L. (2009). Measure-
ment of absorption and scattering with an integrating
sphere detector: Application to microalgae. Journal
of Research of the National Institute of Standards and
Technology, 114(2):69.
Gibson, A. P., Hebden, J. C., and Arridge, S. R. (2005).
Recent advances in diffuse optical imaging. Physics
in Medicine and Biology, 50(4).
Glaser, A. K., Kanick, S. C., Zhang, R., Arce, P., and
Pogue, B. W. (2013). A gamos plug-in for geant4
based monte carlo simulation of radiation-induced
light transport in biological media. Optical Society
of America, 4(5):741–759.
Jacques, S. and Maetzler, C. (2002).
http://omlc.org/software/mie/.
Jacques, S. L. (1996). Origins of tissue optical properties in
the uva visible and nir regions.
Jacques, S. L. (2013). Optical properties of biological tis-
sues: a review. Physics in Medicine and Biology,
58(11):R37.
Morhard, R., Jeffery, H., and McEwan, A. (2014).
Simulation-based optimization of a near-infrared
spectroscopic subcutaneous fat thickness measuring
device. Engineering in Medicine and Biology Soci-
ety (EMBC), 36th Annual International Conference of
the IEEE, pages 510–513.
Sarkar, J. S., Rajput, S., Tripathi, A. K., and Mandal, M.
(2013). Targeted therapy against egfr and vegfr us-
ing zd6474 enhances the therapeutic potential of uv-b
phototherapy in breast cancer cells. Molecular Can-
cer, 12.
van Veen, R. L. P., Sterenborg, H., Pifferi, A., Torricelli,
A., and Cubeddu, R. (2004). Determination of VIS-
NIR absorption coefficients of mammalian fat, with
time-and spatially resolved diffuse reflectance and
transmission spectroscopy. Proc. Biomedical Topical
Meetings, on CD-ROM, Paper SF5, Optical Society of
America, Washington, DC.
Wang, X., Pogue, B. W., Jiang, S., Song, X., Paulsen,
K. D., Kogel, C., Poplack, S. P., and Wells, W. A.
(2005). Approximation of mie scattering parameters
in near-infrared tomography of normal breast tissue in
vivo. Journal of Biomedical Optics, 10(5):051704–
051704–8.
Zhu, C. and Liu, Q. (2013). Review of monte carlo model-
ing of light transport in tissues. Journal of Biomedical
Optics, 18:73–100.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
250
