
EGFR-targeting Peptide Conjugated pH-sensitive Micelles as a
Potential Drug Carrier for Photodynamic Detection and Therapy of
Cancer
Cheng-Liang Peng
1
, Yuan-I Chen
2
, Ying-Hsia Shih
1
, Tsai-Yueh Luo
1
and Ming-Jium Shieh
2
1
Isotope Application Division, Institute of Nuclear Energy Research, Taoyuan, Taiwan
2
Institute of Biomedical Engineering, College of Medicine, National Taiwan University, Taipei, Taiwan
Keywords: Thermosensitive, Photothermal Therapy, Chemotherapy, Micelle, Control Release, Synergistic Effect.
Abstract: Multifunctional theranostics have recently been intensively explored to optimize the efficacy and safety.
Herein, we report multifunctional micelle that constructed from graft copolymer PEGMA-co-PDPA and
diblock copolymer mPEG-b-PCL as the carrier of hydrophobic photosensitizer, chlorin e6 (Ce6) for
simultaneous fluorescence imaging and photodynamic therapy. The functional inner core of PEGMA-co-
PDPA exhibited pH stimulate to accelerate drug release under slightly acidic microenvironments of tumors
and the outer shell of micelles with epidermal growth factor receptor (EGFR)-targeting GE11 peptides for
active targeting of EGFR-overexpressing cancer cells. The results demonstrate that GE11-conjugated
chlorin e6-loaded micelles (GE11-Ce6-micelles) with particle size around 100 nm and the micelles had well
defined core shell structure which was evaluated by TEM. In the in vitro cellular uptake studies, GE11-Ce6-
micelles exhibited a higher amount of intracellular uptake of chlorin e6 in HCT116 cancer cells (EGFR high
expression) via receptor-mediated endocytosis, in contrast with the time-dependent passive uptake of the
non-targeted Ce6-micelles, thereby providing a effective photocytotoxic effect on the HCT116 cancer cells.
In vivo study revealed that GE11-Ce6-micelles exhibited tumor targeting for photodynamic detection and
excellent inhibition on tumor growth after irradiation, indicating that GE11-Ce6-micelles could be
successfully applied to the effective fluorescence imaging and photodynamic therapy of cancer.
1 INTRODUCTION
Photodynamic therapy (PDT) is a novel treatment
for several diseases including age-related macular
degeneration, periodontitis and malignant cancers
(Schmidt-Erfurth and Hasan, 2000). PDT is based on
a photochemical reactions that could produces
localized tissue damage. The activation of
photosensitizers in the target tissues by suitable
wavelengths of light would lead to generations of
reactive oxygen species (ROS) to destroy cancer
cells. Important advantages of PDT over other
therapies include minimal invasiveness, repeated
PDT applicability at the same site, high therapeutic
efficacy, and less side effects in comparison with
other treatments of cancer (Wang et al., 2014a).
Chlorin e6 (Ce6) is a promising photosensitizer
for PDT with an excitation wavelength at 660 nm.
Chlorin e6 exhibits advantageous photophysical
properties including having long lifetimes in its
photoexcited triplet states and high absorption in the
red spectral region that could penetrate tissues
deeper (Wang et al., 2014b). Despite these
significant advantages, Chlorin e6 has poor
solubility in aqueous media and nonspecific
biodistribution with low tumor-targeting efficacy,
which could cause drug loss or photosensitivity in
healthy tissues (Zhang et al., 2003) .
The slight acidic microenvironment of solid
tumor is resulted from their high metabolic rate via
anaerobic glycolysis that causes accumulation of
lactic acid and carbon dioxide. Electrical and
chemical probes show that the pH of the
microenvironment is around 5.8 - 7.2, which is
lower than the physiological pH of 7.4 (Shen et al.,
2008).
To further enhance drug accumulation in tumor
sites while minimizing drug concentration in other
sites, nanoparticles are now being conjugated with
targeting ligands, such as antibodies, proteins,
Peng, C-L., Chen, Y-I., Shih, Y-H., Luo, T-Y. and Shieh, M-J.
EGFR-targeting Peptide Conjugated pH-sensitive Micelles as a Potential Drug Carrier for Photodynamic Detection and Therapy of Cancer.
DOI: 10.5220/0005774201050110
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 2: BIOIMAGING, pages 105-110
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
105

aptamers, and peptides (Lee et al., 2013). Peptides
are small molecules with specific receptor binding,
low immune response induction, and high stability
in vivo. These characteristics of peptides make them
better tumor targeting ligands. The epidermal growth
factor receptor (EGFR) is a cell surface receptor that
is highly expressed in human epithelial cancer cells,
including breast, lung, ovarian, and colon cancers
(Arteaga, 2002).
In this study, the structure and pH sensitivity of
multifunctional micelles were determined and
characterized. The cellular uptake efficiency was
evaluated in HCT116 cells (high EGFR expression)
and SW620 cells (low EGFR expression). At last,
the in vivo tumor-targeted PDT efficacy and
imaging were evaluated in tumor-bearing mice. The
pH-sensitive micelle conjugated with GE11 peptide
is expected to accelerate drug release under slightly
acidic microenvironments of tumors and be
internalized via EGFR-mediated endocytosis
(scheme 1). The goal of this study is to develop a
drug delivery system with enhanced tumor targeting
for cancer therapy.
2 EXPERIMENTAL SECTION
2.1 Synthesis of PEGMA-co-PDPA,
mPEG-PCL, and mal-PEG-PCL
The pH-sensitive copolymer, PEGMA-co-PDPA
(poly(ethylene glycol) methacrylate-co- poly(2-
(diisopropylamino)ethyl methacrylate) was synthesis
by free radical polymerization as described
previously (Peng et al., 2010). Briefly, PEGMA
(0.25 g) and DPA (0.5 g) were added to a flask
equipped with a magnetic stirrer and 5 ml THF. The
AIBN initiator (9.25 mg) was added to the mixture
and the solution was heated at 70 °C for 24 h in an
atmosphere of nitrogen. Unreacted monomers were
removed by dialysis against water for 3 days and the
polymer fraction was lyophilized. Copolymer
compositions were determined with FT-NMR at 500
MHz using chloroform-d (CDCl3) as the solvent.
2.2 Preparation of Chlorin E6-Loaded
Micelles
The blank micelles were prepared by dialysis
method (Chaw et al., 2004). In brief, different
weights (0~5mg) of PEGMA-co-PDPA and mPEG-
PCL were dissolved completely in 0.1N HCL and
then dialysis of base solution for 1 day. Chlorin e6-
loaded micelles (Ce6-micelles) were prepared via
the cosolvent evaporation method (Peng et al.,
2008a). Briefly, 10 mg of PEGMA-co-PDPA, 10mg
of PEG-PCL, and 2mg of mal-PEG-PCL were
dissolved in 0.5 ml THF with Chlorin e6 (1~4mg)
and then added to 5 ml of PBS with stirring at 550
rpm. The organic solvent was evaporated while
being stirred overnight and the remaining portion
was filtered through a 0.22 μm pore size Millex GS
filter to remove non-incorporated drug crystals.
2.3 Preparation of GE11-conjugated
Micelles
EGFR specific peptide (GE11, sequence:
YHWYGYTPQNVI-GGGGC) was used to
established active targeted micelles (Li et al., 2005).
The sequence of “GGGG” as an spacer while the
carboxyl terminal cysteine of the peptide conjugated
with the maleimide of the micelles (Milane et al.,
2010). The conjugation of GE11 were then just
performed by mixture of GE11 and maleimide
containing micelles with different molar ratio at 4 °C
overnight , as described previously (Olivier et al.,
2002). The unconjugated GE11 peptide were
separated by passing through PD-10 desalting
column (GE Healthcare, Uppsala, Sweden).
2.4 Characterization of Chlorin E6-
Loaded Micelles
The mean diameter and PDI of micelles were
determined using a Zetasizer Nano ZS90 apparatus
(Malvern Instruments, Worcestershire, UK). The
size and morphology of micelles were ascertained by
transmission electron microscopy (TEM) using a
model H-7650 microscope (Hitachi, Tokyo, Japan).
To prepare a TEM sample, a drop of sample solution
was placed on a 200-mesh carbon-coated copper
grid and then the excess solution was removed with
filter paper.
The pH sensitivity of micelle was recorded
through size and zeta potential measurements by
dynamic light scattering at various pH values of
PBS.
2.5 In Vivo Fluorescence Imaging
To observe biodistribution of Chlorin e6, female
BALB/c athymic (nut/nut) mice (5-6 weeks old)
were purchased from the National Laboratory
Animal Center (Taipei, Taiwan). HCT-116 cells
(1×106) and SW620 celles (1×106) were inoculated
subcutaneously on the right and left flanks of nude
BIOIMAGING 2016 - 3rd International Conference on Bioimaging
106
mice, respectively. When the tumors reached a
volume of 150 to 200 mm3, mice received an
intravenous injection of Ce6-miccelles or GE11-
Ce6-miccelles (equivalent to 5 mg/kg of Ce6). The
in vivo biodistribution of Ce6 were imaged at 3 and
24 after intravenous injection using an IVIS imaging
system (Xenogen, Alameda, CA, USA). The tumor-
bearing mice were sacrificed 24 h post-injection,
major organs and tumors were excised for isolated
organ imaging to estimate the tissue distribution of
Ce6-miccelles or GE11-Ce6-miccelles.
2.6 In Vivo Photodynamic Therapy
HCT116 cells (1 × 10
6
cells) were implanted
subcutaneously into the right flanks of mice. When
tumors grew to approximately 150-200 mm3 in
volume, 200-500 μl of PBS containing Ce6-micelles
or GE11-Ce6-micelles (equivalent to 5 mg/kg of
Ce6) were injected via tail vein (n = 4 per each
group). At 24 h after injection, tumor tissues were
irradiated by 670 nm diode laser (634 mW/cm2) for
10 min.
3 RESULTS AND DISCUSSION
3.1 Synthesis and Characterization of
Polymers
In this study, the pH-responsive micelles assembled
from mixture of graft copolymer PEGMA-co-PDPA
and diblock copolymer mPEG-PCL were developed
to control drug delivery and enhance the antitumor
efficacy of photodynamic therapy. Synthesis of
PEGMA-co-PDPA and mPEG-b-PCL were carried
out as shown in Figure 1. The potentiometric
measurements was performed to determine the pKa
of the PEGMA-co-PDPA copolymers, each of the
DPA-based copolymers presented a sharp
protonation transition in the pH range 6.0 to 7.0.
According to the pH transition of PEGMA-co-
PDPA, the pH-responsive micelles can deliver
successfully the anticancer drugs to target tumor
tissue but minimize the drug release at normal
tissues. The diblock copolymer, mPEG-PCL were
synthesized by ring-opening polymerization (Peng et
al., 2009).
The micelles ranged from 91.05 to 142.37 nm in
size with various polydispersity indices (Table 1).
Figure 1: Schematic diagram illustrates the fabrication of
GE11-Ce6-micelles.
3.2 Characterization of Chlorin
E6-Loaded Micelles
Chlorin e6 as an photosensitizer was efficiently
encapsulated into the pH-responsive micelles, due to
the hydrophobic interactions between chlorin e6 and
hydrophobic group as DPA or PCL of micelles.
Table 1 lists the results of the loading efficiency,
drug contents, and sizes of the chlorin e6 loaded pH
responsive micelles. After incorporating various
amounts of chlorin e6, all of the samples had a
narrow PDI from 0.109-0.154 and ranged in size
from 96.6-103.1 nm. Chlorin e6-loaded micelles
with a D/P ratio of 1/10 were used and the
encapsulation efficiency was 86.25%. When the
micelles with a D/P ratio of 1/5 or 1/20 were
employed, the encapsulation efficiency was lower
Table 1: Characteristics of micelles.
Sample
D/P
ratio
encapsulation
efficiency (%)
drug content
(%)
Mean size
/nm(PDI)
micelles - - - 91.1 (0.239)
Ce6-
micelles
1/5 75.52 12.09 96.6 (0.109)
Ce6-
micelles
1/10 86.25 7.82 96.7 (0.125)
Ce6-
micelles
1/20 74.35 3.54
103.1
(0.154)
Mal-
micelles
- - -
105.7
(0.222)
Ce6-Mal-
micelles
1/10 85.9 7.81
110.0
(0.184)
GE11-Ce6-
micelles
1/10 78.8 7.16
105.1
(0.293)
EGFR-targeting Peptide Conjugated pH-sensitive Micelles as a Potential Drug Carrier for Photodynamic Detection and Therapy of Cancer
107
than those with a D/P ratio of 1/10. Figure 2 showed
the morphology of the nanoparticles with or without
chlorin e6 as observed by TEM. The images
indicated that micelles with different conditions
were uniform, spherical, and the particle size agreed
with the results measured by dynamic light
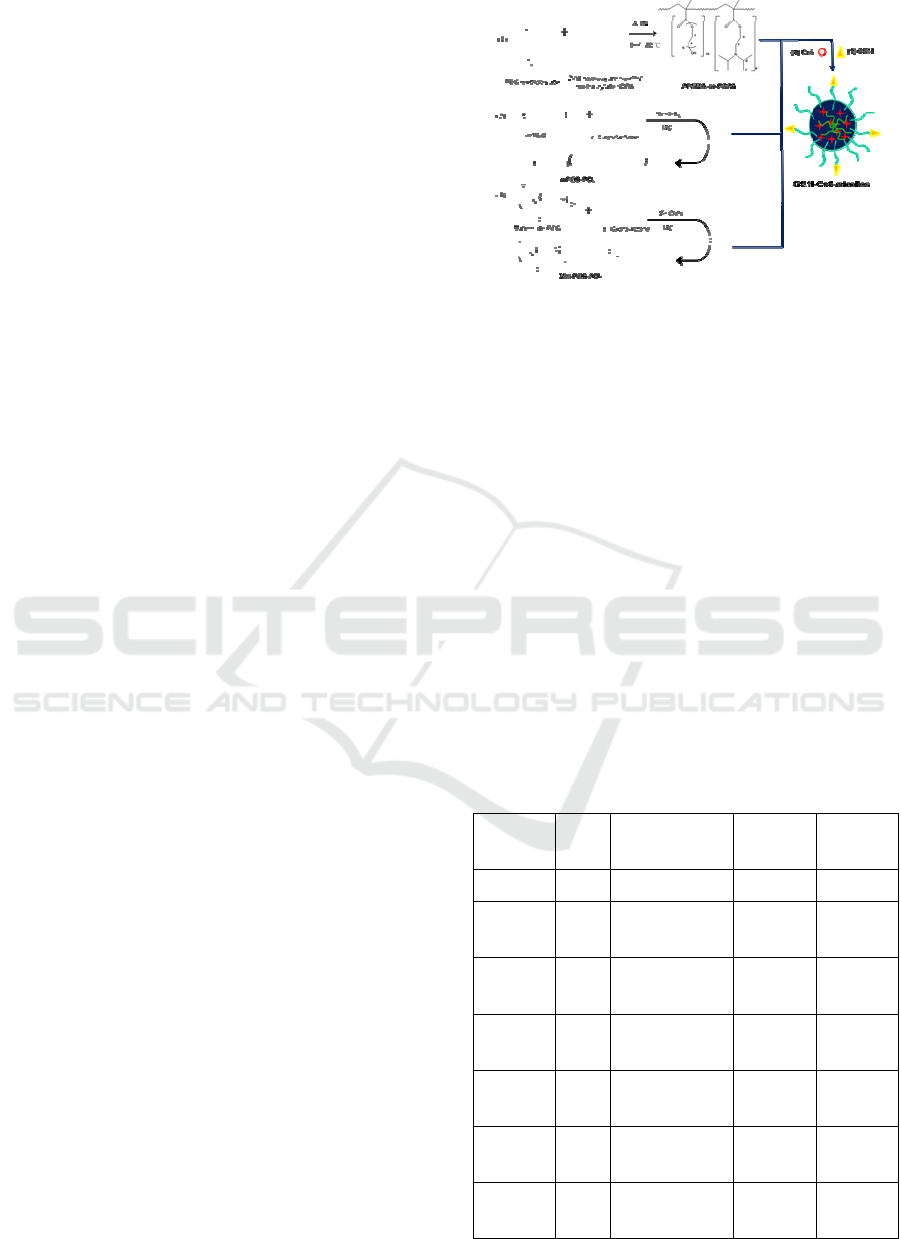
scattering (DLS).Absorbance and fluorescence
spectra of chlorin e6-loaded mixed micelles (Ce6-
micelles), and GE11-conjugated chlorin e6-loaded
mixed micelles (GE11-Ce6-micelles) revealed that
free chlorin e6 exhibited a relatively broad and weak
fluorescence, while the fluorescence of chlorin e6-
loaded mixed micelles was strong and reached a
maximum at 670 nm as show in Figure 3.
3.3 Cellular Uptake and Localization of
Chlorin E6-Loaded Micelles
Subcellular localization of EGFR-targeted GE11-
Ce6-micelles or non-targeted Ce6-micelles were
evaluated by using fluorescence microscopy as
shown in Figure 4. After 5 h incubation, the GE11-
Ce6-micelles were rapidly taken up by the HCT116
cells, as that could be observed from the green
fluorescence of FITC-labeled micelles accumulated
in cells. Intracellular drug release by targeted
micelles and non-targeted micelles could be
projected by red fluorescence of chlorin e6.
Figure 2: Transmission electron micrographs images of (a)
PEGMA-co-DPA micelles, (b) mixed micelles, (c) chlorin
e6 loaded mixed micelles (Ce6-micelles), and (d) chlorin
e6 loaded GE11-conjugated mixed micelles (GE11-Ce6-
micelles).
The fluorescence of GE11-Ce6-micelles
significantly accumulated in the cytoplasm but not in
the nucleus. Furthermore, the most fluorescence of
chlorin e6 in HCT116 celles were colocalized with
the fluorescence of micelles. However, the
fluorescence intensity of chlorin e6 in HCT116 cells
remarkably decreased when cells treated with GE11-
Ce6-micelles and excess amount of free GE11
peptides, indicated that the specific uptake of GE11-
Ce6-micelles into tumor cells through EGFR-
mediated endocytosis.
3.4 In Vivo Fluorescence Imaging
To compare the in vivo biodistribution of EGFR-
targeted GE11-Ce6-micelles or non-targeted Ce6-
micelles (equivalent to 5mg/kg of chlorin e6) was
injected intravenously into HCT116 (high
expression EGFR) and SW620 (low expression
EGFR) tumor-bearing mice. The in vivo
biodistribution of chlorin e6 could be directly
monitored by non-invasive and real-time
fluorescence imaging of the whole body, because
chlorin e6 can emit strong near infrared (NIR)
fluorescence for efficient tracking (Koo et al., 2010).
Figure 3: (a) Absorbance spectra of free chlorin e6 (Ce6),
chlorin e6-loaded mixed micelles (Ce6-micelles), and
GE11-conjugated chlorin e6-loaded mixed micelles
(GE11-Ce6-micelles) (b) fluorescence spectra of GE11-
Ce6-mixed micelles in difference pH values of buffers, (c)
fluorescence spectra of chlorin e6-loaded mPEG-b-PCL
micelles in difference pH values of buffers (equivalent to
2 μg/ml of chlorin e6). The fluorescence spectra were
measured with an excitation of 403 nm and emissions in
the 600–800 nm range. (d) Singlet oxygen generation of
GE11-Ce6-micelles in various pH Tris buffer solutions.
Figure 4: Subcellular localization of chlorin e6 in HCT116
cells treated with EGFR-targeted GE11-Ce6-micelles or
non-targeted Ce6-micelles.
BIOIMAGING 2016 - 3rd International Conference on Bioimaging
108
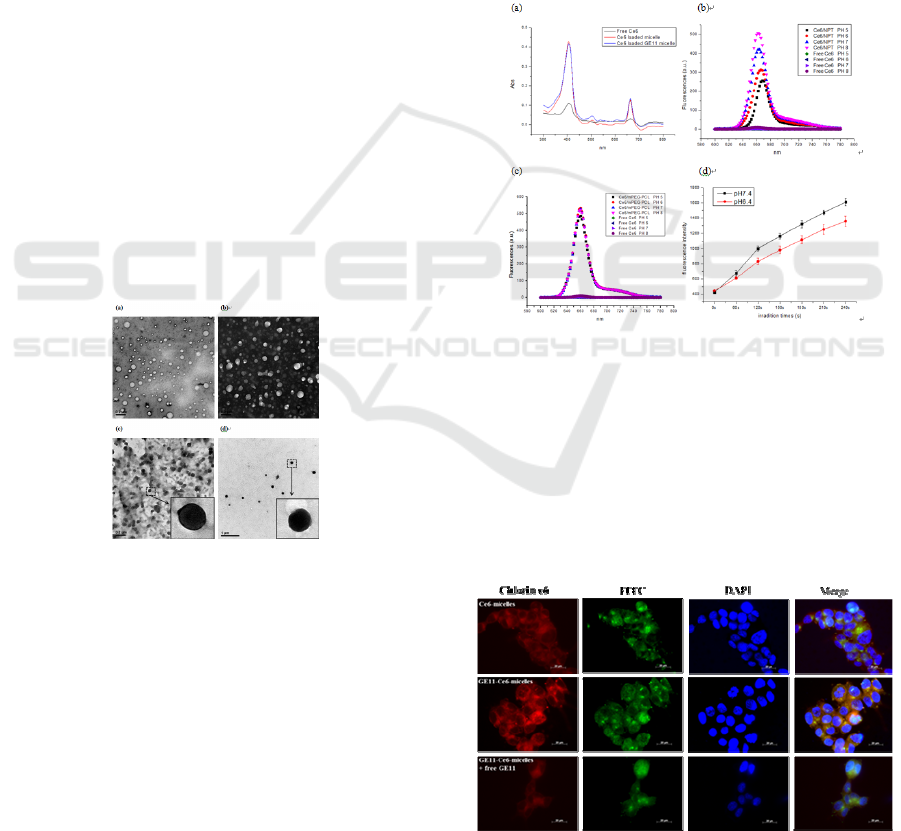
The results demonstrated that GE11-Ce6-micelles
were effectively accumulated in the HCT116 tumor,
compared to non-targeted mixed micelles (Figure 5).
At 3 h post-injection, significant fluorescence
emitted from the GE11-Ce6-micelles injected mice
was observed in the HCT116 tumor but not SW620
tumor.
Figure 5: In vivo and ex vivo fluorescence imaging of
HCT116 and SW620 tumor-bearing mice administrated
with EGFR-targeted GE11-Ce6-micelles and non-targeted
Ce6-micelles. (a) Whole body fluorescence images of
tumor-bearing mice treated with GE11-Ce6-micelles and
Ce6-micelles. Arrows indicate tumor sites. (b) Ex vivo
fluorescence images of organs and tumors were acquired
after 24 h injection of GE11-Ce6-micelles. The total
fluorescent photon counts of chlorin e6 in (c) HCT116
tumor and (d) SW620 tumor in the corresponding
fluorescence images in Figure 8a was quantified.
Meanwhile, there are also fluorescence signal in
the other tissues. At 24 h post-injection, the
fluorescence of GE11-Ce6-micelles was maintained
in HCT116 tumor, indicating that it were not subject
to rapid excretion from the mice. Additionally, the
total fluorescent photon counts of chlorin e6 in
tumor with GE11-Ce6-micelles was about 2-2.5 fold
higher than that with Ce6-micelles at 3 h and 24h
post-injection in HCT116 tumor.
3.5 In Vivo Photodynamic Therapy
In vivo photodynamic therapeutic efficacy of GE11-
Ce6-micelles or Ce6-micelles (equivalent to 5mg/kg
of chlorin e6) with laser irradiation was evaluated by
measuring tumor growth in HCT116 tumor-bearing
mice. After 24h injection, the tumors were irradiated
with a red laser (670 nm, 634 mW/cm2) for 10 min
and the tumor size was monitored for 22 days.
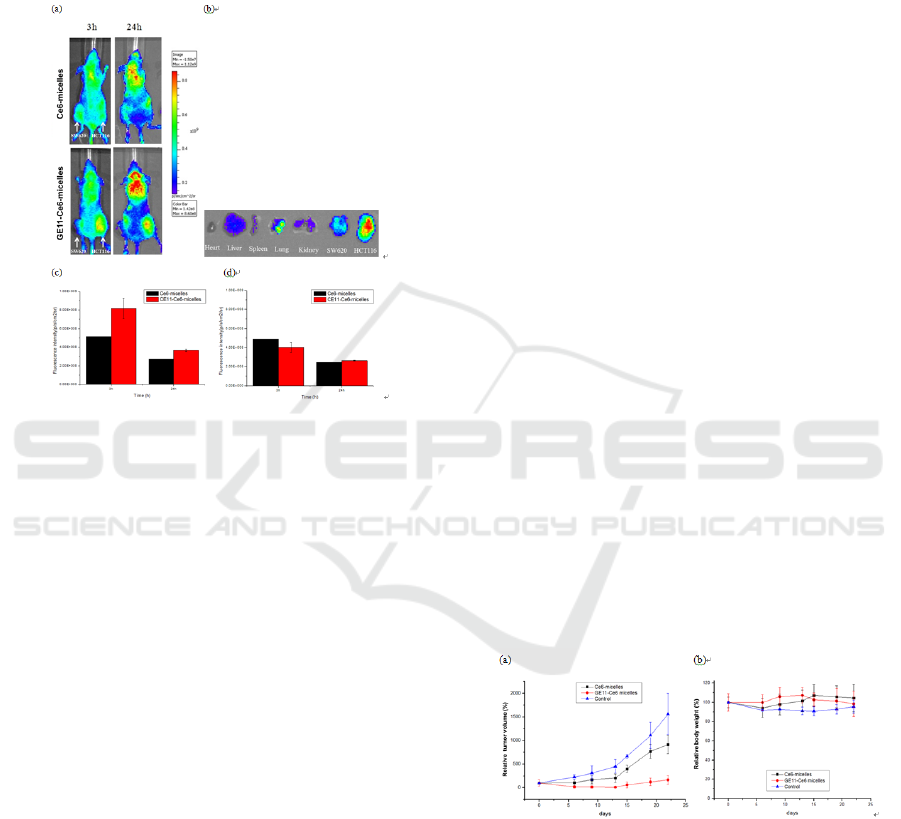
Figure 6a showed the therapeutic efficacy of each
treatment was monitored by evaluating the relative
tumor volume for 22 days. The PDT mediated by
GE11-Ce6-micelles reduced relative tumor volume
compared with control tumors and tumors treated
with Ce6-micelles plus laser irradiation.. The body
weight were not significantly different between
control mice and PDT-treated mice, indicating that
photodynamic therapy mediated by Ce6-micelles or
GE11-Ce6-micelles did not result in unacceptable
toxicity(Figure 6b).
4 CONCLUSIONS
We have prepared and characterized pH-resposive
micelle constructed from graft copolymer PEGMA-
co-DPA and diblock copolymer mPEG-b-PCL as
drug delivery carrier for simultaneous photodynamic
imaging and therapy. In vitro and in vivo studies
confirmed that EGFR-targeted o GE11-Ce6-micelles
enhanced specific uptake by cancer cells via receptor
mediated endocytosis pathway and improved PDT
of EGFR overexpressing cancer cells. Moreover, the
tumor targeted delivery of GE11-Ce6-micelles
allowed detection of EGFR tumors by near infrared
imaging. In tumor-bearing mice models, GE11-Ce6-
micelles could effectively suppress the tumor growth
compared to non-targeted Ce6-micelles. In
conclusion, GE11-Ce6-micelles could be
successfully applied to fluorescence imaging and
effective photodynamic therapy of cancer.
Figure 6: In vivo photodynamic therapeutic efficacy of
Ce6-micelles or GE11-Ce6-micelles in HCT116 tumor-
bearing mice. (a) The tumor volumes and (b) body weights
were measured during the 22-day evaluation period in
mice were treated with PBS (Control), Ce6-micelles plus
laser irradiation, or GE11-Ce6-micelles plus laser
irradiation. Data indicate means and standard errors.
EGFR-targeting Peptide Conjugated pH-sensitive Micelles as a Potential Drug Carrier for Photodynamic Detection and Therapy of Cancer
109

REFERENCES
Arteaga, C. L. 2002. Epidermal Growth Factor Receptor
Dependence In Human Tumors: More Than Just
Expression? The Oncologist, 7, 31-39.
Chaw, C.-S., Chooi, K.-W., Liu, X.-M., Tan, C.-W.,
Wang, L. & Yang, Y.-Y. 2004. Thermally Responsive
Core-Shell Nanoparticles Self-Assembled From
Cholesteryl End-Capped And Grafted
Polyacrylamides:: Drug Incorporation And In Vitro
Release. Biomaterials, 25, 4297-4308.
Huang, P., Xu, C., Lin, J., Wang, C., Wang, X. S., Zhang,
C. L., Zhou, X. J., Guo, S. W. & Cui, D. X. 2011.
Folic Acid-Conjugated Graphene Oxide Loaded With
Photosensitizers For Targeting Photodynamic
Therapy. Theranostics, 1, 240-250.
Koo, H., Lee, H., Lee, S., Min, K. H., Kim, M. S., Lee, D.
S., Choi, Y., Kwon, I. C., Kim, K. & Jeong, S. Y.
2010. In Vivo Tumor Diagnosis And Photodynamic
Therapy Via Tumoral Ph-Responsive Polymeric
Micelles. Chemical Communications, 46, 5668-5670.
Lee, P.-C., Chiou, Y.-C., Wong, J.-M., Peng, C.-L. &
Shieh, M.-J. 2013. Targeting Colorectal Cancer Cells
With Single-Walled Carbon Nanotubes Conjugated To
Anticancer Agent Sn-38 And Egfr Antibody.
Biomaterials, 34, 8756-8765.
Li, Z., Zhao, R., Wu, X., Sun, Y., Yao, M., Li, J., Xu, Y.
& Gu, J. 2005. Identification And Characterization Of
A Novel Peptide Ligand Of Epidermal Growth Factor
Receptor For Targeted Delivery Of Therapeutics. The
Faseb Journal, 19, 1978-1985.
Milane, L., Duan, Z. & Amiji, M. 2010. Development Of
Egfr-Targeted Polymer Blend Nanocarriers For
Combination Paclitaxel/Lonidamine Delivery To Treat
Multi-Drug Resistance In Human Breast And Ovarian
Tumor Cells. Molecular Pharmaceutics, 8, 185-203.
Min, K. H., Kim, J.-H., Bae, S. M., Shin, H., Kim, M. S.,
Park, S., Lee, H., Park, R.-W., Kim, I.-S., Kim, K.,
Kwon, I. C., Jeong, S. Y. & Lee, D. S. 2010. Tumoral
Acidic Ph-Responsive Mpeg-Poly(Β-Amino Ester)
Polymeric Micelles For Cancer Targeting Therapy.
Journal Of Controlled Release, 144, 259-266.
Olivier, J.-C., Huertas, R., Lee, H. J., Calon, F. &
Pardridge, W. M. 2002. Synthesis Of Pegylated
Immunonanoparticles. Pharmaceutical Research, 19,
1137-1143.
Peng, C.-L., Shieh, M.-J., Tsai, M.-H., Chang, C.-C. &
Lai, P.-S. 2008a. Self-Assembled Star-Shaped
Chlorin-Core Poly(Ɛ-Caprolactone)–Poly(Ethylene
Glycol) Diblock Copolymer Micelles For Dual
Chemo-Photodynamic Therapies. Biomaterials, 29,
3599-3608.
Peng, C.-L., Yang, L.-Y., Luo, T.-Y., Lai, P.-S., Yang, S.-
J., Lin, W.-J. & Shieh, M.-J. 2010. Development Of
Ph Sensitive 2-(Diisopropylamino)Ethyl Methacrylate
Based Nanoparticles For Photodynamic Therapy.
Nanotechnology, 21, 155103.
Peng, C. L., Lai, P. S., Lin, F. H., Wu, S. Y. H. & Shieh,
M. J. 2009. Dual Chemotherapy And Photodynamic
Therapy In An Ht-29 Human Colon Cancer Xenograft
Model Using Sn-38-Loaded Chlorin-Core Star Block
Copolymer Micelles. Biomaterials, 30, 3614-3625.
Peng, C. L., Shieh, M. J., Tsai, M. H., Chang, C. C. & Lai,
P. S. 2008b. Self-Assembled Star-Shaped Chlorin-
Core Poly(C-Caprolactone)-Poly(Ethylene Glycol)
Diblock Copolymer Micelles For Dual Chemo-
Photodynamic Therapies. Biomaterials, 29, 3599-
3608.
Schmidt-Erfurth, U. & Hasan, T. 2000. Mechanisms Of
Action Of Photodynamic Therapy With Verteporfin
For The Treatment Of Age-Related Macular
Degeneration. Survey Of Ophthalmology, 45, 195-
214.
Shen, Y., Tang, H., Radosz, M., Van Kirk, E. & Murdoch,
W. 2008. Ph-Responsive Nanoparticles For Cancer
Drug Delivery. In: Jain, K. (Ed.) Drug Delivery
Systems. Humana Press.
Wang, M., Chen, Z., Zheng, W., Zhu, H., Lu, S., Ma, E.,
Tu, D., Zhou, S., Huang, M. & Chen, X. 2014a.
Lanthanide-Doped Upconversion Nanoparticles
Electrostatically Coupled With Photosensitizers For
Near-Infrared-Triggered Photodynamic Therapy.
Nanoscale, 6, 8274-8282.
Wang, X., Liu, K., Yang, G., Cheng, L., He, L., Liu, Y.,
Li, Y., Guo, L. & Liu, Z. 2014b. Near-Infrared Light
Triggered Photodynamic Therapy In Combination
With Gene Therapy Using Upconversion
Nanoparticles For Effective Cancer Cell Killing.
Nanoscale.
Zhang, G.-D., Harada, A., Nishiyama, N., Jiang, D.-L.,
Koyama, H., Aida, T. & Kataoka, K. 2003. Polyion
Complex Micelles Entrapping Cationic Dendrimer
Porphyrin: Effective Photosensitizer For
Photodynamic Therapy Of Cancer. Journal Of
Controlled Release, 93, 141-150.
BIOIMAGING 2016 - 3rd International Conference on Bioimaging
110
