
Magnetic Resonance Imaging at 7 Tesla with Dedicated
Radiofrequency Coils
Application to Cervical Cord and Knee
Maria Evelina Fantacci
1,2
, Laura Biagi
3
, Mirco Cosottini
4,5
, Mauro Costagli
3,5
, Massimo Marletta
6
,
Alessandra Retico
2
, Riccardo Stara
1,2,7
, Mark Symms
8
, Gianluigi Tiberi
3,5
, Virna Zampa
6
and Michela Tosetti
3,5
1
Dipartimento di Fisica, Università di Pisa, Largo Pontecorvo 3, Pisa, Italy
2
Istituto Nazionale di Fisica Nucleare (INFN, Sez. Pisa), Pisa, Italy
3
IRCCS Stella Maris, Pisa, Calambrone, Pisa, Italy
4
Dip. di Ricerca Traslazionale e delle Nuove Tecnologie in Medicina e Chirurgia, Univ. di Pisa, Pisa, Italy
5
Fondazione IMAGO7, Pisa, Italy
6
Dipartimento di Radiologia Diagnostica ed Interventistica AOUP, Pisa, Italy
7
Lucas center for Imaging, Department of Radiology, Stanford University, Stanford, CA 94305, U.S.A.
8
General Electric ASL (EMEA), Pisa, Italy
Keywords: Ultra High Field Magnetic Resonance Imaging Coils, Cervical Cord UHF MRI, Knee UHF MRI.
Abstract: Magnetic Resonance (MR) Imaging is a valuable tool in the diagnosis and monitoring of various
musculoskeletal pathologies. New Ultra-High Field (UHF) 7 T MRI systems, with their enhanced Signal-to-
Noise Ratio, may offer increased image quality in terms of spatial resolution and/or shorter scanning time
compared to lower field systems. However, these benefits can be difficult to obtain because of increased
radio-frequency (RF) inhomogeneity, increased Specific Absorption Rate (SAR) and the relative lack of
specialized and commercially available RF coils compared to lower field systems. This study reports the
feasibility of imaging in bones and cartilages at UHF with a 7 T MR scanner available at the IMAGO7
Foundation (Pisa, Italy). Dedicated radio-frequency coils for proton imaging have been designed,
developed, optimized for different anatomical regions and validated in vivo, and are now ready for clinical
research studies. The performance of the RF coil prototypes in targeting different anatomical regions are
also demonstrated, obtaining images of the neck (the cervical cord) and of the knee (trabecular bone and
cartilages).
1 INTRODUCTION
The current research in the field of Magnetic
Resonance (MR) biomedical imaging (MRI) is
moving towards increasingly higher static magnetic
field strengths. Whereas 3 Tesla scanners are
becoming widespread in clinical applications,
scanners working at higher static magnetic fields are
currently available only at a limited number of
laboratories in the world, and only for research
purposes.
There are about 60 MR systems for human
studies operating at 7 Tesla or above worldwide, and
they have already demonstrated the great capability
and potential of Ultra-High Field (UHF) MR, and
many technical challenges remain (Ugurbil, 2003;
Kraff, 2015).
The IMAGO7 Foundation in Pisa (Italy) owns
and manages the first and only 7 Tesla whole-body
MR scanner (950-MR scanner, GE Medical
Systems, Milwaukee, WI) in Italy. In this
framework, a research collaboration between the
IMAGO7 Foundation and the Italian National
Institute for Nuclear Physics (INFN) aims to develop
important hardware components, such as RF coils
for specific MR applications, and to exploit the UHF
Fantacci, M., Biagi, L., Cosottini, M., Costagli, M., Marletta, M., Retico, A., Stara, R., Symms, M., Tiberi, G., Zampa, V. and Tosetti, M.
Magnetic Resonance Imaging at 7 Tesla with Dedicated Radiofrequency Coils - Application to Cervical Cord and Knee.
DOI: 10.5220/0005774102290234
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 229-234
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
229

potential in several research areas, including MSK
imaging.
Among the clinical applications that will benefit
from the improved resolution and signal-to-noise
ratio (SNR) obtainable at high magnetic field is the
muscoloskeletal (MSK) system. UHF MR imaging
of the MSK system, including small joints, offers
important potential advantages over lower field
systems in increased sensitivity and enhanced
contrast. However, these benefits can be difficult to
obtain because of increased radio-frequency (RF)
inhomogeneity, increased Specific Absorption Rate
(SAR) and the relative lack of specialized and
commercially available RF coils compared to lower
field systems.
A number of in-vivo studies of the
musculoskeletal (MSK) system at high field
strengths have already been carried out (Majundar,
2008; Farooki, 2002; Gambarota, 2007; Regatte,
2007; Krug, 2009), including the investigation of the
possibility to perform sequential studies during the
clinical and instrumental follow up of
neuromuscular disorders (Retico, 2015). The latter
can be useful for a variety of applications: a) to
allow an earlier diagnosis also in asymptomatic
patients; b) to improve the monitoring of the
progression of muscle involvement; c) to provide
valuable information on the efficacy of ongoing
therapeutic studies (drugs, gene or stem cell
therapy), representing a possible alternative to serial
muscle biopsies. Moreover, at UHF, for this
application fat suppression should be used to reduce
the chemical shift artifacts between fat and water
frequencies.
This paper presents two RF coil prototypes
suitable for 7T MSK applications targeting the
geometry of different anatomical regions (neck and
knee) based on quadrature Tx/Rx surface RF coil
suitable for the detection of the proton signal and the
first images acquired in vivo by means of these
dedicated RF coil prototypes. In fact, dedicated neck
coils are fundamental for studying the spinal cord in
several pathologies of the central nervous system
such as multiple sclerosis, or myelopathies of
different origin. In particular the high resolution and
SNR of the UHF can be exploited to investigate the
gray matter and the fiber bundles within the spinal
cord of patients with amyotrophic lateral sclerosis.
Moreover, UHF MRI of the knee (Krug, 2009) can
allow an accurate characterization of morphology
and biochemical quality of the cartilages for clinical
assessment of early pathological conditions of
cartilage in osteoarthritis, and can allow the
quantification of trabecular bone architecture useful
for clinical assessment of osteoporosis.
This paper is organized as follows: first, the
choice of the coil design is motivated according to
the available MR system and the anatomical region
under investigation; then, the choice of the hardware
components and the coil construction details are
provided; finally the results, consisting of the first in
vivo images acquired on human subjects, are
presented.
2 MATERIALS AND METHODS
2.1 Quadrature Tx/Rx Surface RF
1
H
Coils for 7 T MRI
The availability of commercial RF coils for UHF
MR systems is still limited. Therefore, UHF research
sites often set up RF laboratories to develop suitable
coils for specific applications.
The choice of the RF coil design depends on the
target anatomy, on the desired MR acquisition
modality (e.g. MRI, MR spectroscopy (MRS),
multinuclear MRS, …) and it is constrained by the
available MR system equipment.
This study focuses on the adult human neck (for
the cervical cord applications) and knee (for
trabecular bone and cartilage applications). Two
coils have been designed, with geometrical
optimization for accommodating an adult human
neck and an adult human knee, respectively.
The MR system available at the IMAGO7
Foundation is a 7 T scanner currently equipped with
two channels for transmission, which can be either
both for proton (i.e.
1
H, I and Q channels) or one for
proton and one for another nucleus (i.e.
1
H and MNS
channel).
As a linearly polarized surface coil for proton
MRI is expected to suffer transmit field
inhomogeneity problems typical at UHF, in an
attempt to obtain more informative structural images
a specific coil design has been used. It consists of a
quadrature surface coil, where the two channels are
both used for proton.
For both the prototypes the coil housing is
designed using the AutoCAD CAD package; the
supports have to be designed according to the target
anatomy, and has to be comfortable, easy to use and
safe. To this purpose we left 10 mm between the
inner surface (where the coil circuits will lie) and the
outer layer (where the neck or the knee will be
positioned). Concerning the choice of the materials,
we used for the mechanical support a polycarbonate
structure obtained by 3D printing.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
230

The coil circuits consist of a printed circuit board
(PCB) in FR4, board thickness 200 micron, copper
thickness 35 micron. The circuits are covered by a
protective paint to avoid oxidation.
The coils presented here are constituted of two
partially overlapped loops driven in quadrature. The
shape of the mechanical frame of the RF coil is a
semi-cylindrical saddle of inner radius 70 mm and
outer radius 85 mm.
The support of the neck coil presents a flat
portion to best accommodate the neck lying on the
coil in the supine position while the support of the
knee coil presents a hemi-cylindrical geometry
(Figure 1).
The RF circuit consists of two loops with
dimensions 170 mm along the horizontal axis, 60
mm and 130 mm along the vertical axial for the
central and peripheral part, respectively. The two
loops are geometrically decoupled by a partial
overlap of 18 mm (adjustable). Referring to Figure
2, the list of components used for assembling the
coil is given in Table 1.
Figure 1: The quadrature Tx/Rx surface RF coil suitable
for the detection of proton signal in the neck (left) and in
the knee (right).
Figure 2: Circuit of the quadrature
1
H RF surface coil
made by two square loops with partial overlapping.
Table 1: RF components of the single-tuned quadrature
1
H
RF coil.
CT
1
6.8 pF
CT
2,5
5.1 pF
CT
3,4,6,7
4.2 pF
CMS 22 pF
CMP 1.0 pF
The tuning procedure of the
1
H loop is performed
after measuring the inductance, which implies the
following operation: 1) a known capacitor has been
added to the loop; 2) the correspondent resonance
frequency has been measured through a Vector
Network Analyzer (VNA, E5071C, Agilent
Technologies); the inductance is determined by
analytical calculation. Next, the loop has been tuned
to 298.03 MHz, i.e. the Larmor frequency of the
1
H
at 7 T. Next, the coil has to be matched: matching
can be achieved either with or without a load, i.e. in
the unloaded/loaded condition. A capacitive
matching with load is performed; the Q factor is
calculated through appropriate VNA measurements,
and the corresponding resistance is derived
(Mispelter, 2006). The matching capacitor was
determined by using a Smith Chart procedure
(Smith, 1995). The workbench measurements
provided the following values: matching < -17 dB
on both channels, coupling < -15 dB. The Q factors
are 120 and 20 for both channels, in loaded and
unloaded conditions, respectively. The simulated B
1
+
and E maps obtained for unitary input power of 1
kW are shown in Figure 3, which demonstrates that
quadrature operation of the 7 T surface coil
improves B
1
+
field homogeneity with respect to a
linearly polarized surface coil.
Figure 3: Simulated B
1
+
(a) and E (b) field maps of the
quadrature
1
H RF surface coil for unitary input power of
1kW. Measured B
1
+
(c) for the same input power.
Magnetic Resonance Imaging at 7 Tesla with Dedicated Radiofrequency Coils - Application to Cervical Cord and Knee
231
2.2 Human Image Acquisitions
Six healthy and one pathological (alteration of the
patellar cartilage) volunteers were considered for the
preliminary acquisitions reported here. Healthy
volunteer age ranged between 24 to 61 years, the
pathological volunteer was 62 years old. In our
Institute clinical studies follow the ethical guidelines
of our local ethics committee. Informed written
parental consent was obtained before enrollment in
the study. As expected, any side effect from muscle
MRI examination has not observed.
Morphological images have been acquired by
means of 3D MERGE and 3D FIESTA sequences,
optimized taking into account the relaxation times of
the tissues of interest at 7 Tesla. In order to evaluate
also the biochemical behaviour of the cartilage in the
pathological subject, T2 and T2* maps (Krug, 2009)
have been also computed.
3 RESULTS
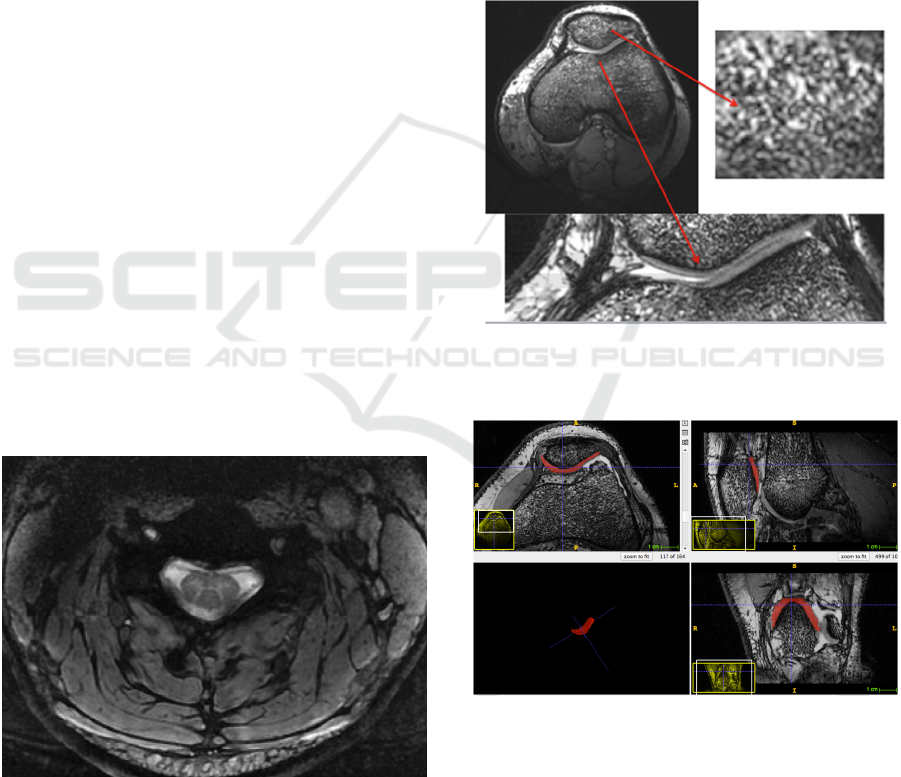
The neck RF coil was used to obtain images of the
neck and the cervical cord in healthy subjects
(Figure 4).
The sequence used to obtain the image reported
in Fig. 4 was a 3D MERGE with 0.5 mm in-plane
resolution, TR = 30 ms, TE = 17.5 ms, thickness =
2.2 mm.
The image obtained demonstrates that this coil
provides excellent anatomical images of the cervical
spine, where spinal gray matter and white matter can
be clearly depicted.
Figure 4: Cervical cord of a healthy volunteer acquired at
7 T with the quadrature
1
H surface RF coil and a 3D
MERGE sequence. Note the high quality of the image
with a clear depiction of the H shape of the spinal grey
matter.
The knee RF coil was used to obtain images of
the knee in healthy volunteers, in order to assess
both the architecture of the trabecular bone and the
morphology of the cartilage (Figure 5). The
sequence used to obtain the image reported in Fig. 5
was a 3D FIESTA with 0.156 mm in-plane
resolution, FA = 20, TR = 6.3 ms, TE = 2.5 ms,
thickness = 0.8 mm.
The patellar cartilage was then segmented by
means of the ITK-SNAP (Yushkevich, 2006)
software tool, obtaining the results reported in
Figure 6 (in the three axial planes and in the 3D
volume rendering). Then the volume of the
segmented cartilage has been quantified, obtaining
the result of 1779 mm
3
.
Figure 5: Knee of a healthy volunteer in which clearly
depicts the trabecular architecture of the bone and the
cartilages.
Figure 6: Segmentation and 3D rendering of the patellar
cartilage of a healthy volunteer.
The knee RF coil was also used to obtain images
of the knee in the pathological volunteer, in which
the pathological change is clearly evident.
The sequence used to obtain the image reported
in Figure 7 (left knee) was a 3D FIESTA with 0.156
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
232
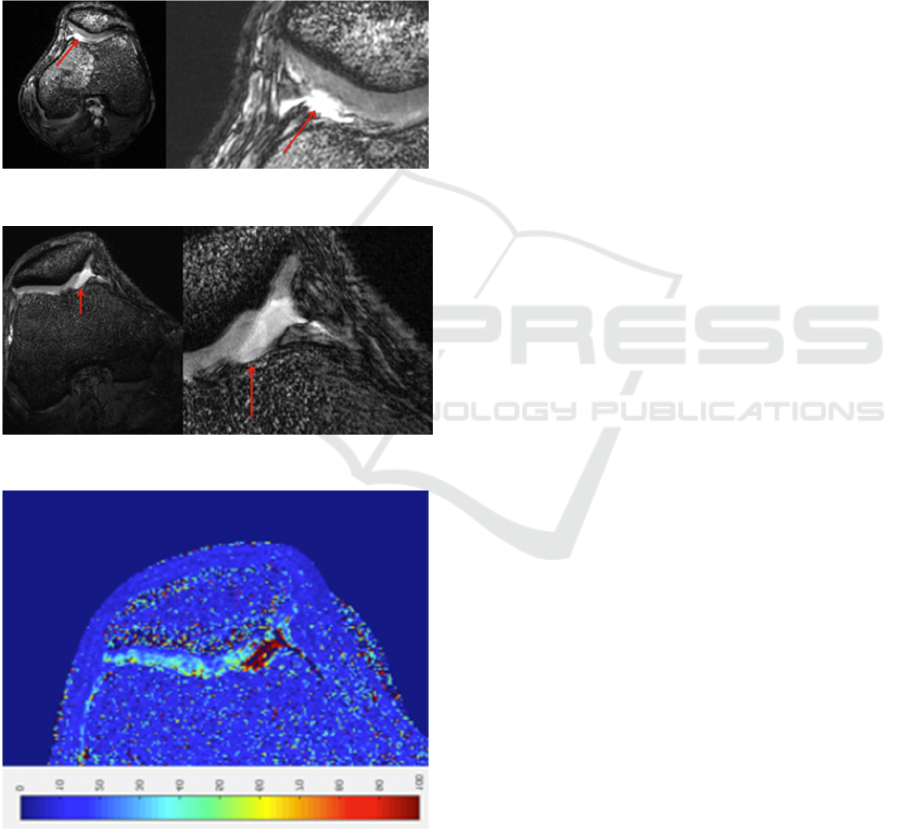
mm in-plane resolution, FA = 20, TR = 6.3 ms, TE =
2.4 ms, thickness = 0.8 mm.
The sequence used to obtain the image reported
in Figure 8 (right knee) was a 3D FIESTA with
0.156 mm in-plane resolution, FA = 20, TR = 9.0
ms, TE = 3.4 ms, thickness = 0.8 mm. In Figure 9 is
reported an example of T2* map calculation of the
same slice of Figure 8, realized by means of a 3D
MERGE sequence with 6 echo times. In these
images, of the right knee, the lack of cartilage in the
medial facet of the patella and other alterations of
the cartilage are clearly highlighted.
Figure 7: Left knee of the pathological volunteer.
Figure 8: Right knee of the pathological volunteer.
Figure 9: T2* map of the right knee of the pathological
volunteer.
4 CONCLUSIONS
We presented the recent achievements in human
MRI with a 7 T MR whole-body scanner. We
designed and developed dedicated RF surface coils
for
1
H imaging. The RF coil prototype for neck has
been validated in vivo on healthy volunteers. The
obtained results in the cervical spinal cord
demonstrate a high image quality and if confirmed
in a larger sample of patients might constitute a
promising tool in exploring a complex anatomical
region that is prone to susceptibility artifact at UHF.
The RF coil prototype for knee has been validated in
vivo on healthy and pathological volunteers. Due to
the low amount of water in the bone and the small
size of the cartilage, this district is well suitable for a
study using UHF. With this technology it was
possible to carry out a thorough assessment both
morphological and functional. The obtained results
demonstrate that the research in trabecular bone and
cartilages characterization, comprising quantitative
assessment of cartilage volume and evaluation of
biochemical behaviour, can take advantage from
UHF MR with dedicated coils.
REFERENCES
Farooki, S, C.J. Ashman, CJ et al., 2002. “In vivo high-
resolution MR imaging of the carpal tunnel at 8.0
Tesla,” Skeletal Radiology, vol. 31(8), pp. 445-450.
Kraff, O, Fischer, A et al., 2015. “MRI at 7 Tesla and
Above: Demonstrated and Potential Capabilities,”
Journal of Magnetic Resonance Imaging, vol. 41, pp.
13–33.
Krug, R et al., 2009. “Imaging of the Musculoskeletal
System In Vivo Using Ultra-high Field Magnetic
Resonance at 7 T”, Investigative Radiology, vol. 44 n.
9, pp. 613-618.
Gambarota, G, Veltien, A, et al., 2007. “Magnetic
resonance imaging and T2 relaxometry of human
median nerve at 7 Tesla,” Muscle Nerve, vol. 36(3),
pp. 368-373.
Majumdar, S, 2008. “Ultra-High Field-7 Tesla Imaging of
the Musculoskeletal System,” ISMRM High Field
Workshop, Rome.
Mispelter, J, Lupu M et al., 2006. “NMR Probeheads for
Biophysical and Biomedical Experiments: Theorical
Principles Pratical Guidilines,” Imperial College Press.
Retico A, Stara, R et al., 2015. “Non-invasive assessment
of Neuromuscular Disorders by 7 tesla Magnetic
Resonance Imaging and Spectroscopy: Dedicated
radio-frequency coil development”, 2015 IEEE
International Symposium on Medical Measurements
and Applications (MeMeA) pp. 68-73.
Magnetic Resonance Imaging at 7 Tesla with Dedicated Radiofrequency Coils - Application to Cervical Cord and Knee
233

Regatte, RR and Schweitzer, ME, 2007. “Ultra-high field
MRI of the muscoloskeletal system at 7.0 T,” Journal
of Magnetic Resonance Imaging, vol. 25(2), pp. 262-
269.
Smith, P, 1995. Electronic Applications of the Smith
Chart, 2nd edition, 1995, SciTech/Noble Publishing,
Raleigh, North Carolina.
Ugurbil, K, Adriany, G et al., 2003. P. Andersen, et al.,
“Ultrahigh field magnetic resonance imaging and
spectroscopy,” Magn. Reson. Imaging, vol. 21, pp.
1263-1281, 2003.
Yushkevich, PA, Piven, J et al., 2006. “User-guided 3D
active contour segmentation of anatomical structures:
Significantly improved efficiency and reliability.”
Neuroimage vol. 31, n. 3, pp 1116-28.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
234
