
Electrochemical Analysis of Accelerated Aging of PEDOT-PTS
Coated Screen-printed Electrodes
Nathalia Peixoto
1
, Tjerignimin Silue
1
, Catalina Vallejo-Giraldo
2
and Manus Biggs
2
1
Electrical and Computer Engineering, George Mason University, Fairfax, VA, U.S.A.
2
Centre for Research in Medical Devices (CURAM), National University of Ireland Galway, Galway, Ireland
Keywords: Implantable Electrodes, Electrode Coating, Stability of Electrode Coatings, Electrochemical Impedance
Spectroscopy, Accelerated Aging, Cyclic Voltammetry, Long-term Electrode Testing.
Abstract: We have developed a deposition method that enhances charge delivery of screen printed electrodes by up to
six times through electrochemical deposition of poly (3,4-ethylenedioxythiophene):p-toluenesulfonate
(PEDOT-PTS). In order to elucidate the effects of PEDOT-PTS deposition on the long-term electrochemical
characterization of screen-printed electrodes we characterized electrode stability with cyclic voltammetry and
impedance spectroscopy at room temperature and at 47 °C. A deposition current of 0.4 mA/cm
2
guarantees
coverage of the working electrode conductive area with no spill of the conductive polymer through the
insulating tracks. Control electrodes show charge storage capacity of 0.25 mC. PEDOT-PTS deposited
electrodes are stable for over 4 months and present cathodic charge storage capacity of 1.25 mC.
1 INTRODUCTION
The electrode interface continues to be the main
puzzle piece in the development of neural coupled
devices and implanted sensors. Current challenges are
focused on addressing the accumulation of
hypertrophic astrocytes, and the development of
electrode-investing glial scar tissue (Vallejo-Giraldo
et al., 2014), which increases impedance and prevents
electrode integration with excitable neural tissues.
The development of next-generation electrode
technologies is informed by the biological and
physico-mechanical considerations of nervous tissues
and neural interfaces (Fernandez-Yague, 2015),
which has led to advances in the fabrication of high-
density microelectrode arrays (Green et al., 2013) and
the development of biochemically modified
(Kikkawa et al., 2014) and mechanically biomimetic
neuroelectrode systems (Ware et al., 2012).
Long-term recording with implanted electrode
systems in non-human primates represents a
significant bottleneck in the development of brain-
computer-coupled devices and of novel medical
solutions to neural disorders. Although long-term
neural recording has been reported in a handful of
studies (Nicolelis et al., 2003) using traditional
electrode approaches (parylene-coated microwires),
the controversy of long-term neural recording
proliferates due to confounding variables such as
handling of the implant, chemical and mechanical
properties of implants, surgical technique, and quality
of materials implanted, among other fabrication
variables.
In order to investigate the mechanisms of
electrode failures, in vitro experiments are performed
with a subset of those variables, under controlled
environmental conditions. Previous research from our
group, for example, showed that parylene as an
insulator would age within 4 months if implanted.
However, when parylene is applied in conjunction
with ALD (atomic layer deposition) alumina, it
demonstrated a four-fold increase in lifetime
(Minnikanti et al., 2014). Furthermore, we compared
these two kinds of insulations while testing them at
relatively high temperatures, or under “accelerated
aging” conditions, which allowed us to reliably
predict the lifetime of this polymer in vivo.
Poly(3,4-ethylenedioxythiophene) (PEDOT) has
gained much attention recently (Green et al., 2013,
Kim et al., 2014, Mandal et al., 2014), given its
biocompatibility, versatility in terms of counter-ion
species and high charge storage capacity (Green et al.,
2013). Our group has previously investigated several
conductive polymers, as well as surface modifications
for biological applications (Fernandez-Yague et al.,
76
Peixoto, N., Silue, T., Vallejo-Giraldo, C. and Biggs, M.
Electrochemical Analysis of Accelerated Aging of PEDOT-PTS Coated Screen-printed Electrodes.
DOI: 10.5220/0005700600760082
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 76-82
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2014) and in particular for the development of smart
neuroelectrode applications (Mokarian-Tabari et al.,
2015).
We hypothesized that we could apply the same
principles of testing the material lifetime of electrodes
coated with poly(3,4-ethylenedioxythiophene):p-
toluenesulfonate (PEDOT-PTS) and that
electrodeposition of this conducting polymer at the
recording surface would enhance their
electrochemical properties. In a previous report we
have shown that PEDOT was not as stable as iridium
oxide when used for stimulation (Peixoto et al.,
2009). Here we will address a PEDOT formulation
with stable counter-ions and leverage accelerated
aging and electrochemical characterization to
demonstrate stability of the superficial layers.
In this manuscript we report on the lifetime
assessment of such coatings when used for macro
screen-printed electrodes. In order to determine
stability, we electrodeposit PEDOT-PTS on
commercially available low-cost electrodes with a
carbon layer as the active material. Those strips, as
well as control strips, were subjected to cyclic
voltammetry and electrochemical impedance analysis
for up to one month at room temperature, and
thereafter at elevated temperatures (in our case, 47
°C) for up to three months. The charge delivery
capacity of the electrodes is then evaluated, relative
to their initial value, and the robustness of coatings
determined based on that parameter and on the
stability of the modulus and phase angle in impedance
profiles.
2 METHODS
Here we describe the utilized substrates, the methods
of electrodeposition of PEDOT-PTS, and the
electrochemical methods that were utlized in order to
characterize the stability of the coatings and of non-
coated control samples.
2.1 Substrates and Solutions
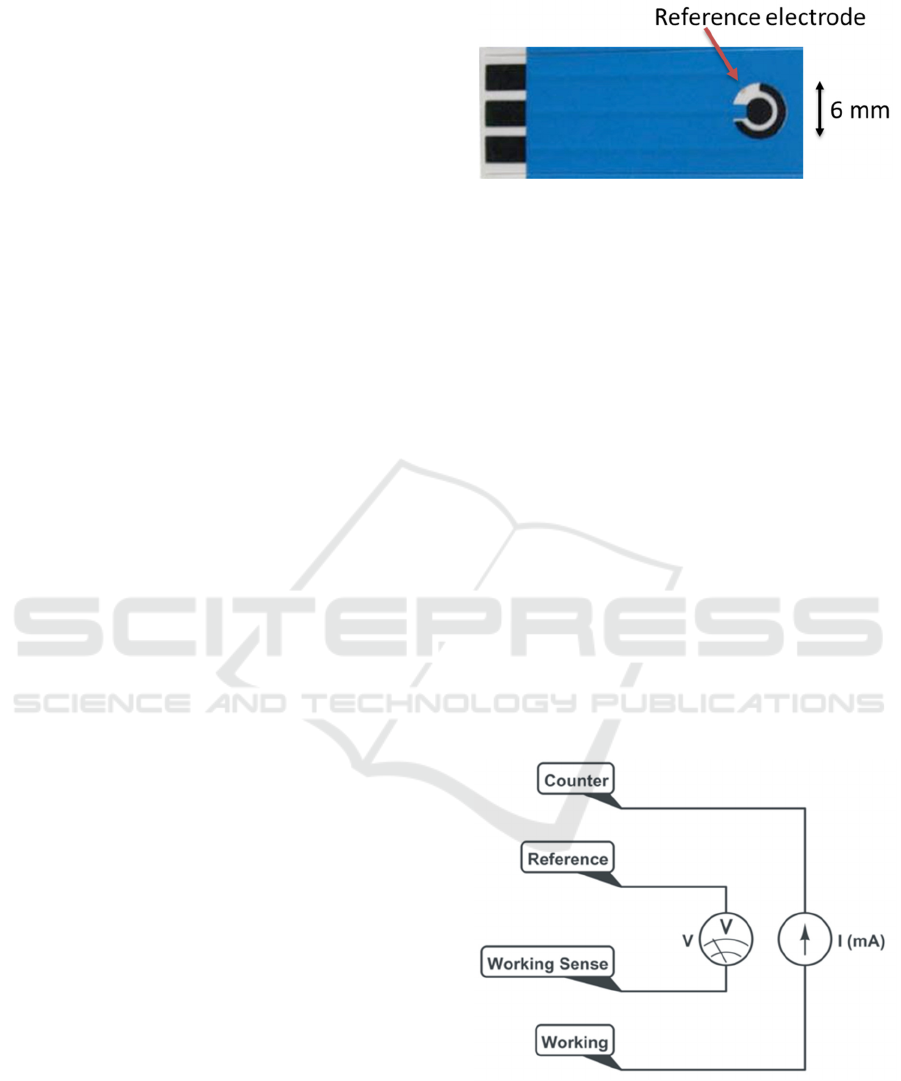
Screen-printed paper-based electrodes (Zensor,
TE100) were acquired from CH Instruments. Figure
1 shows the as-received electrodes. The counter and
working electrode are carbon-based, while the
reference electrode is a silver-silver chloride
formulation.
All characterizations were performed with
electrodes immersed in phosphate buffered saline
(Sigma-Aldrich, St. Louis, MO) at 7.4 pH.
Figure 1: As-received screen-printed electrodes. The active
areas are carbon black and silver/silver-chloride. The tabs
on the left allow for electrical connection through alligator
clips. The active areas are the ring (6mm outer diameter),
the center circle (3 mm diameter), and the reference
electrode, silver/silver-chloride. The Ag/AgCl is identified
by the red arrow. An electrically insulating polymeric cover
is identified in blue.
2.2 Electrochemical Deposition
The electrodeposition of PEDOT-PTS films was
conducted under ambient conditions. A solution of
0.05 M EDOT (Sigma Aldrich, Ireland) and 0.1 M
PTS (Sigma Aldrich, Ireland) was prepared in a 50:50
vol% mixture of acetonitrile and DI water. The
electrolyte solution containing the monomeric EDOT
was placed in an in-house fabricated electrochemical
cell system, as shown in Figure 2. The cell was
connected to a Princeton Applied Research
electrochemical potentiostat/galvanostat model 2273.
The electrochemical apparatus consisted of a four
electrode set-up and galvanostatic electrodeposition
was performed with 0.03 and 0.64 mA, and
providing current densities of 0.4 and 9.014 mA cm
-2
Figure 2: Four-electrode set-up for electrodeposition of
PEDOT-PTS onto Zensor TE100 electrodes under
galvanostatic conditions. Current is first adjusted according
to electrode surface area and then it is applied between
working and counter electrodes, while voltage is measured
between the sensor (working sense) and reference
electrodes.
Electrochemical Analysis of Accelerated Aging of PEDOT-PTS Coated Screen-printed Electrodes
77
respectively, over a constant electrodeposition time of
450 seconds. When the deposition was completed,
each electrode was soaked in deionized water for 24
h to remove excess electrolyte and unreacted EDOT
monomer.
Figure 3 shows magnified images of working
electrode following PEDOT-PTS electrodeposition.
In this manuscript we discuss results pertaining to the
0.4 mA/cm
2
current deposition, as the higher current
showed polymeric coating of the plastic insulation.
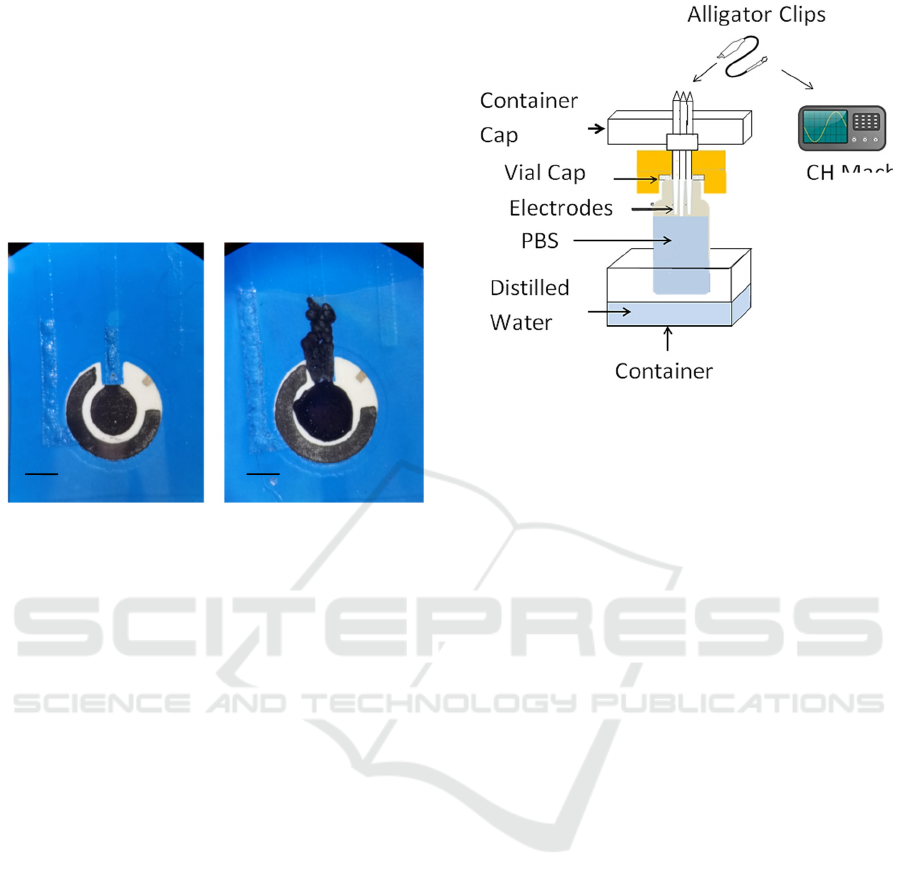
Figure 3: As-deposited screen-printed electrodes. PEDOT-
PTS coated electrodes using a current density of 0.4 mA
cm
-2
(left) and PEDOT-PTS coated electrodes using a
current density of 9.014 mA cm
-2
(right). These magnified
images show details of the surface of the counter and
working electrodes immediately after deposition.
Horizontal scale: 3 mm.
2.3 Characterization Techniques
Control electrodes (carbon-based) as well as PEDOT-
deposited electrodes were immersed in PBS and
characterized over time at 24 °C (room temperature)
and at 47 °C. The temperature controller was filled
with Aluminium beads and temperature was logged
over time with a temperature data-logger.
Temperature variation from the heated experiments
(47 °C) was within 1 °C over the course of
experiment. A common problem with long-term
experiments is the evaporation of the media. We
resolved evaporation issues as follows: (a) dental
cement was used on the cap of vials; (b) Teflon tape
used around the threads; (c) a wet environment was
created inside a beaker in order to raise the water
vapor pressure and to allow for temperature control
through a bead bath. Figure 4 shows a schematic of
the setup built and used throughout the
characterization experiments.
Electrochemical characterization was performed
using a 16 channel multiplexer attached to a
CHI660D potentiostat (CH instruments, Bee Cave,
TX). The potentiostat, the electrodes, and all cables
were kept inside a Faraday cage. The potentiostat is
Figure 4: The experimental setup used for long-term
characterization of coated and control electrodes. Dental
cement was used to seal screen-printed electrodes into
electrode cells following PEDOT-PTS deposition. In order
to prevent evaporation, vials were filled with phosphate
buffered saline. Electrodes were inserted through the vial
caps and secured with dental cement. Alligator clips and
multi-stranded wires were used to contact the three
electrodes to the potentiostat, and the whole vial was kept
inside a container with distilled water in order to prevent
evaporation. An external environment with saturated
humidity guaranteed that no evaporation took place over
three months.
connected to a PC, kept outside the cage, through a
USB cable. Cyclic voltammetry (CV) is an
electrochemical method that entails the application of
a voltage between the counter and the working
electrode from -0.7 V to 0.7 V while measuring the
current through the working electrode, in reference to
the silver/silver-chloride electrode. While this test is
usually performed continuously, here we ran five
periods, at a scan rate of 50 mV/s. Each electrode
therefore undergoes five CV cycles and then one EIS
test. We have the capability of running up to 16
channels, but we only used 6 of those (3 controls and
3 experimental strips) in order to demonstrate the
stability of the coatings for up to two months.
Once a voltammetry cycle was recorded, the current
was integrated over half of the period of the voltage
applied (for example, from -0.7 V to 0.7 V, or from
0.7 V to -0.7 V) and the charge transferred could be
obtained. Because this test was done at low scan rates,
it was possible to calculate the charge storage
capacity of the electrode. More specifically, because
integration was applied over the cathodic half of the
period, the resulting plot can be referred to as the
cCSC (cathodic charge storage capacity), given in
Coulombs.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
78
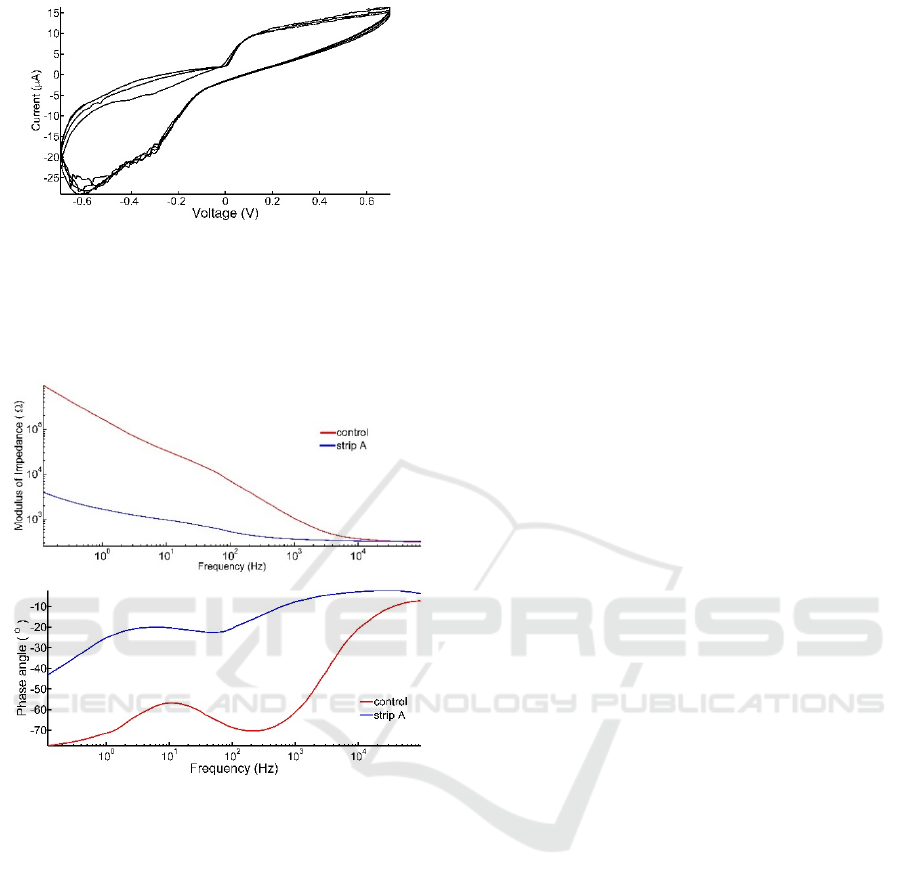
Figure 5: Five cycles of voltammetric spectra of control
non-coated carbon electrodes characterized at room
temperature, in PBS, with a three-electrode setup, voltage
range from -0.7 V to 0.7 V. Current is shown in micro
Amperes. Cyclic voltammetry profile was stable for over
one week (data not shown).
Figure 6: Electrochemical impedance spectroscopy of
control and coated electrodes characterized at room
temperature, in PBS, with a three-electrode setup. Both
impedance modulus (top graph) and phase angle (bottom
graph) show a typical profile for carbon black in the control
electrode (red curves). For lower frequencies, the
electrochemical cell is capacitive (with angles approaching
90 degrees), while for higher frequencies the characteristic
resistive phase is seen. This profile was stable over one
week (testing period, data not shown). Strip A, in blue,
shows lower impedance modulus and a more resistive phase
angle than the control electrode.
Electrochemical impedance spectroscopy (EIS)
was implemented with a 20 mVrms sinusoidal
voltage signal with frequency varying from 0.1 Hz to
100 kHz. Both the modulus and phase were then
recorded for one sweep of frequency. Subsequently
the electrode was subjected to another cyclic
voltammetry (CV) and impedance spectroscopy
(EIS) cycle. Throughout 24 hours at least 80 cycles of
these two tests were recorded.
Figure 5 shows a typical CV spectrum for a
control electrode. Following one week a similar
profile was obtained.
While the CV shows how much charge an
electrode could deliver, the impedance profile
facilitated the generation of the characteristic circuit
component that better describes the interface between
the coating and the electrolyte. Figure 6 shows the
capacitive nature of the carbon-electrolyte interface at
lower frequencies, and the resistive interface at higher
frequencies.
3 RESULTS
“As-received” non-coated electrodes (n=10) were
used as control substrates in order to determine the
stability of the cathodic charge storage capacity. This
was calculated as a function of the surface area of the
working electrode (7 mm
2
), and found to be
0.25±0.10 mC. Due to excessive PEDOT-PTS
deposition on the insulated regions when using a
current density of 9.014 mA cm
-2
,
we have
characterized further only electrodes coated with
PEDOT-PTS electrodeposited with a current density
of 0.4 mA cm
-2
, which were assessed for long-term
stability in vitro.
PEDOT-PTS films deposited at a current density
of 0.4 mA cm
-2
were immersed in PBS and analysed
at room temperature for one month, along with three
control strips. During this time, approximately 2,000
runs were performed on each electrode. In order to
analyze the impedance changes of each electrode, EIS
runs for each experimental condition were combined
and a mean plot obtained. Figure 7A shows an
example of a twenty-four hour interval, for PEDOT-
PTS coated electrodes. The stability of the modulus
of impedances over time is within one fold for low
frequencies (up to 1 Hz) and it is statistically
insignificant for frequencies above 10 Hz. Figures 7A
and 7B also demonstrate the effects of PEDOT-PTS
deposition on the modulus and phase of the low
frequency impedance spectrum, reducing the
modulus of impedance to less than 10 kΩ at
frequencies less than 1 Hz. This is in conjunction with
a 30 degree angle on the phase of impedance,
indicating a more resistive electrode-electrolyte
interface.
While the EIS results demonstrated repeatable
profiles, the CV spectra presented noise at voltages
above 0.4 V and below -0.4 V. However, the profiles
were significantly different to those of the non-coated
carbon control electrodes (Figure 4), indicating an
Electrochemical Analysis of Accelerated Aging of PEDOT-PTS Coated Screen-printed Electrodes
79
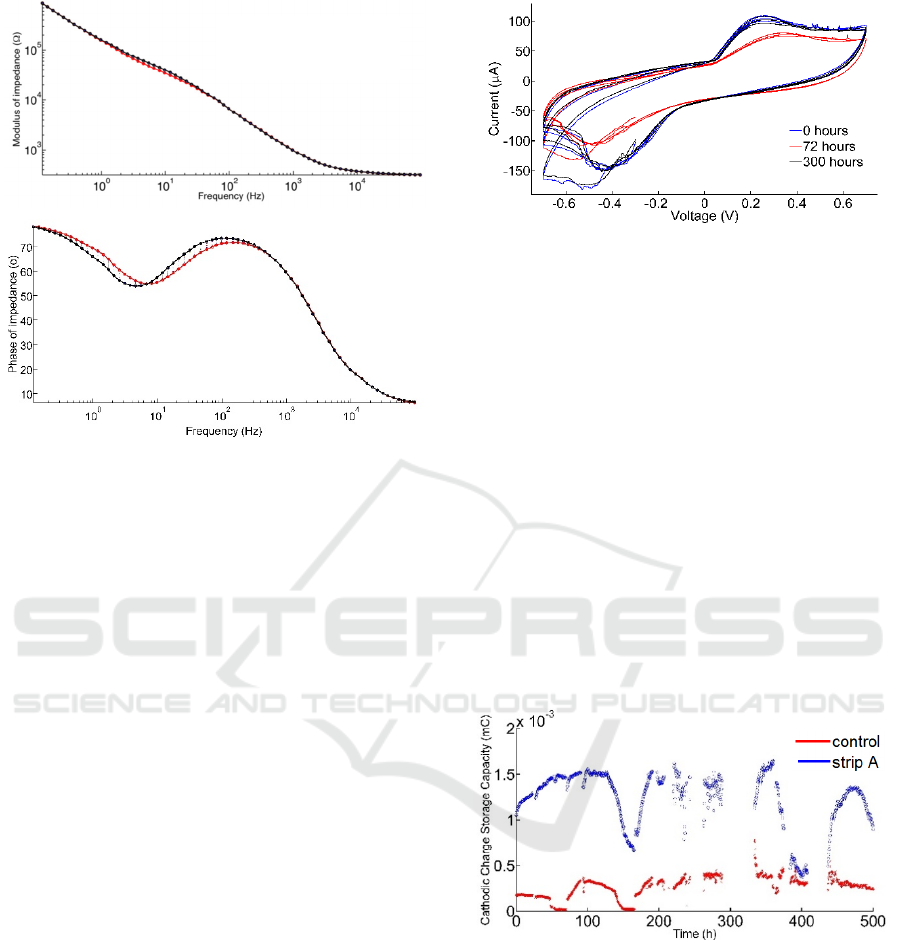
Figure 7: Overlay of electrochemical impedance
spectroscopy measurements (modulus) and of phase
measurements for PEDOT-PTS coated electrodes. The
measurements were obtained at t=0 and after 24 hours at
room temperature, in PBS, with a three-electrode setup. The
modulus of impedance was stable across all frequencies
tested (0.1 Hz through 100 kHz) (top graph). The phase was
observed to increase by up to 10 degrees between the
beginning and end of the test (lower graph).
increased charge transfer capacity (over 1 mC at room
temperature), a higher surface area, and a more
electrically active material. Figure 8 shows CVs taken
at three different times during a 600-hour run at 47°C.
Offsets in current are not considered when calculating
the cathodic charge storage capacity. In order to
measure cCSC the average current per cycle of the
voltage is first subtracted from the current.
After one month of electrochemical analysis at room
temperature, both controls and PEDOT-PTS coated
electrodes presented profiles which are not
statistically different from the profiles recorded
during the initial tests (as shown in figure 7 for
impedance profiles). In order to force the aging of the
electrodes, the temperature of electrolyte was
increased to 47 °C and the cathodic charge storage
capacity was assessed for up to two months. At this
elevated temperature, the main qualitative difference
noted was electrical noise. The impact on the EIS and
CV however was minimal. In order to quantify
stability, we measured the cCSC for over 1,500
voltammetric cycles. Figure 9 shows a summary of
these plots obtained from PEDOT-PTS coated and
non-coated electrodes. The standard deviation was
not plotted on this graph for clarity.
Figure 8: Cyclic voltammetry for PEDOT-PTS coated
electrodes characterized at 47°C. Electrodes were
immersed in PBS, and the CV recorded using a three-
electrode setup, with a scan rate of 50 mV/s. The
voltammetry was performed against the reference electrode
potential. The blue curve was taken at 0 h (first cycle
recorded), red curve at 72 hours, and black curve at 144
hours.
Each electrode was characterized approximately 3
times per hour, for up to 500 hours. There were two
interruptions due to power outages, one around the
300-hour mark, and one around the 420-hour mark.
This means that we are reporting on approximately
400 hours (non-consecutive). Approximately 1,000
files were generated per electrode and each files was
used to extract one cCSC, which was then plotted
along the time axis (Figure 9). The mean and standard
deviation of the cCSCs for control and PEDOT-PTS
electrodes was recorded as 0.26±0.12 mC; 1.25±0.30
mC, respectively.
Figure 9: Cathodic charge storage capacity over time for
control (red) and PEDOT-PTS coated electrodes (blue and
black). The charge storage capacity indicates the stability
of the cyclic voltammetry through a combined mean data
point. Each number corresponds to a full cycle of a CV
performed on the electrode from -0.7 V to 0.7 V at 50 mV/s,
against a reference electrode. These experiments were
performed in PBS held at 47°C.
4 DISCUSSION
Recently, several groups have demonstrated the
t=0 (black)
t=24h (red)
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
80

successful deposition of PEDOT-PTS on Platinum
substrates for electrical stimulation in neural
prosthetics applications (Green et al., 2012, Green et
al., 2013) or as conductive films electro-sprayed onto
glass substrates (Kim et al., 2104). To our knowledge,
this is the first report of a PEDOT-PTS film
successfully deposited on Carbon substrates, on
commercially available screen-printed electrodes.
We have successfully designed a coating protocol
for PEDOT-PTS thin films that can be adjusted in
order to obtain varying charge delivery capacities.
The charge storage capacity of the films showed a
five to seven fold increase over the commercially
available carbon coating, and given the surface area
of the working electrode (7 mm
2
), and the highly non-
uniform surface, the cathodic charge storage capacity
of 0.25±0.10 mC was not surprising. A usual side
effect of thin film coatings, and in particular for
PEDOT electrodes, is the splitting of layers, also
known as delamination, over time. Factors that affect
delamination are the dynamic range of the voltage
applied during CV tests, the temperature at which the
characterizations are performed, the surface
roughness and the electrostatic interaction at the
material interface.
Delamination can usually be visualized on the
surface of the electrode seen as cracks when it is
substantial (Green et al., 2012), and it is a significant
concern in implantable applications since coating
technologies can be important to preserve
biocompatibility during chronic recording or
stimulation (Vallejo-Giraldo et al.). Critically, when
during the onset of delamination, electrochemical
methods can point to subtle or microscopic defects
that are not readily found microscopically. With the
combination of cyclic voltammetry and impedance
spectroscopy profiles, it was possible to ascertain
over time, the stability of the interface between the
working electrode or the electrode coating and an
electrolyte.
By characterizing electrodes over several months
we demonstrated that the charge and the impedance
can vary around a mean value but remained stable in
vitro. Robustness of the PEDOT-PTS could then be
demonstrated at room temperature and when
subjected to accelerated aging at elevated
temperatures. In can be hypothesized that for
applications utilizing implantable PEDOT-PTS
coated electrodes, a similar robust behavior can be
expected. The original motivation for electrochemical
analysis at 47 °C was derived from the hypothesis that
in general, for every 10 degree increase in
temperature, it can expected that the lifetime of the
polymer will decrease by a factor of two. In other
words, given that 10 days is the mean time to failure
cited in the literature for implanted PEDOT-PTS
coatings (Green et al, 2012), it could be expected that
the carbon-based electrodes investigated in this study
would fail within 5 days.
The robustness of the screen-printed electrodes
was a further unexpected result observed in this
exploration and the polymeric insulating coating did
not delaminate over the course of three months (total
test time) indicating the potential applications of these
cost-effective devices for implantable devices.
Future studies will focus on the miniaturization of
the deposition area and on validating the stability of
PEDOT-PTS coated electrodes in neuronal-glial
culture maintained for over three months, while
leveraging the electrodes for stimulation and
recording of extra-cellular activity.
5 CONCLUSIONS
PEDOT-PTS films, when deposited on Carbon-based
substrates, enhance the electrode-electrolyte interface
through increasing the charge delivery with a constant
surface area. The potential of this coating approach
for neuroelectrode applications is further validated
through the coating persistence and delamination was
not observed for up to two months in age-accelerated
conditions. We intend to test miniaturized electrodes
with the same coating process, and with neuronal-
glial cultures, in order to further characterize
PEDOT-PTS for biological applications.
ACKNOWLEDGEMENTS
We acknowledge the participation of our summer
students, Kevin Luu (UCSD) and Jose Pahuacho
Palomino (GMU), who performed some of the
experiments discussed here. M.J. Biggs is a Science
Foundation Ireland, Starting Investigator SIRG
COFUND fellow (grant agreement no.
11/SIRG/B2135), and a funded investigator through
the Science Foundation Ireland Centre for Research
in Medical Devices (CÚRAM) (Grant agreement no.
13/RC/2073).
REFERENCES
Fernandez-Yague, Marc A., et al. "Biomimetic approaches
in bone tissue engineering: integrating biological and
physicomechanical strategies." Advanced drug delivery
Electrochemical Analysis of Accelerated Aging of PEDOT-PTS Coated Screen-printed Electrodes
81

reviews 84 (2015): 1-29.
Green, Rylie A., et al. "Substrate dependent stability of
conducting polymer coatings on medical electrodes."
Biomaterials 33.25 (2012): 5875-5886.
Green, R. A., et al. "Performance of conducting polymer
electrodes for stimulating neuroprosthetics." Journal of
neural engineering 10.1 (2013): 016009.
Kikkawa, Y. S., T. Nakagawa, L. Ying, Y. Tabata, H.
Tsubouchi, A. Ido and J. Ito (2014). "Growth factor-
eluting cochlear implant electrode: impact on residual
auditory function, insertional trauma, and fibrosis." J
Transl Med 12: 280.
Kim, Seul-Gi, et al. "Highly conductive PEDOT: PTS films
interfacially polymerized using electro spray deposition
and enhanced by plasma doping." Japanese Journal of
Applied Physics 53.3 (2014): 035501.
Mandal, Himadri S., et al. "Improving the performance of
poly (3, 4-ethylenedioxythiophene) for brain–machine
interface applications." Acta biomaterialia 10.6 (2014):
2446-2454.
Minnikanti, Saugandhika, et al. "Lifetime assessment of
atomic-layer-deposited Al2O3–Parylene C bilayer
coating for neural interfaces using accelerated age
testing and electrochemical characterization." Acta
biomaterialia 10.2 (2014): 960-967.
Mokarian-Tabari, P., C. Vallejo-Giraldo, M. Fernandez-
Yague, C. Cummins, M. A. Morris and M. J. P. Biggs
(2015). "Nanoscale neuroelectrode modification via
sub-20 nm silicon nanowires through self-assembly of
block copolymers." Journal of Materials Science-
Materials in Medicine 26(2).
Nicolelis, Miguel AL, et al. "Chronic, multisite,
multielectrode recordings in macaque monkeys."
Proceedings of the National Academy of Sciences
100.19 (2003): 11041-11046.
Peixoto, Nathalia, et al. "Charge storage: stability measures
in implantable electrodes." Engineering in Medicine
and Biology Society, 2009. EMBC 2009. Annual
International Conference of the IEEE. IEEE, 2009.
Vallejo-Giraldo, Catalina, Adriona Kelly, and Manus JP
Biggs. "Biofunctionalisation of electrically conducting
polymers." Drug discovery today 19.1 (2014): 88-94.
Ware, Taylor, et al. "ThreeDimensional Flexible
Electronics Enabled by Shape Memory Polymer
Substrates for Responsive Neural Interfaces."
Macromolecular materials and engineering 297.12
(2012): 1193-1202.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
82
