
Smart Sensing System for the Detection of Specific Human Motion
Symptoms of the Parkinson’s Disease
A. Kita
1
, P. Lorenzi
1
, G. Romano
1
, R. Rao
1
, R. Parisi
1
, A. Suppa
2
, M. Bologna
2
, A. Berardelli
2
and F. Irrera
1
1
Department of Information Engineering, Electronics and Communications, Sapienza University of Rome,
Via Eudossiana 18, Rome, Italy
2
Department of Neurology and Psychiatry, Sapienza University of Rome, Rome, Italy
Keywords: Wearable Wireless Inertial Sensors, Motion Features, Freezing of Gait, Neural Network Algorithm, Time
based Analysis, Parkinson’s Disease, Rhythmic Auditory Stimulation.
Abstract: We propose two different wearable wireless sensing systems based on Inertial Measurement Units for the
home monitoring of specific symptoms of the Parkinson’s disease. In one configuration just one sensor is
inserted in a headset, in the other configuration two sensors are positioned on the patient’s shins. They
recognize and classify noticeable motion disorders potentially dangerous for patients and give an audio
feedback. The systems use dedicated algorithms for real time processing of the raw signals from
accelerometers and gyroscopes, one of which is based on an artificial neural network and another on a time-
based analysis. The headset system detects satisfactorily a wide class of motion irregularities including the
trunk disorders, but is poorly reliable on Parkinson’s patients. The other system with sensors on the shins
provides an early detection of the freezing of gait with excellent performance in terms of sensitivity and
precision, and timely provides a rhythmic auditory stimulation to the patient for releasing the involuntary
block state.
1 INTRODUCTION
A wide variety of movement disorders and gait
irregularities are typical symptoms of the Parkinson
Disease (PD) (Nieuwboer et al., 2001). Among
others, the freezing of gait (FOG) is a really disabling
one. FOG is paroxysmal block of movements, which
takes place in an advanced stage of the PD if the
patient is not properly covered by the therapy. During
the FOG, patients refer that their feet are “stuck to the
ground” (Spildooren et al, 2010). In this situation, the
patients make attempts to make a step, oscillating and
thrusting forward the trunk, which can cause
catastrophic events as falls (Bloem et al, 2004). Often,
the FOG is anticipated by a progressive step
shortening (pre-freezing state) (J. Spildooren et al.,
2010), after which the patient stops completely. It has
been shown that a rhythmic auditory stimulation
(RAS) can lead the patients out of the FOG state (P.
Arias and Cudeiro, 2010). The possibility to provide
a RAS timely at the onset of the symptom or in the
pre-freezing state would avoid the undesired
consequences of the block. During the last few years,
several different systems for the automatic detection
of the FOG have been proposed. These are based on
the classification of electrical signals coming from
inertial sensors properly positioned on the patient
body (Lorenzi et al., 2015), (Mazilu et al., 2014), (
Bachlin et al., 2010), (Moore et al., 2013), (B.
Sijobert et al., 2014), (Mazilu et al., 2013) (Cola et
al., 2015), (Atallah et al., 2014). In our work, we
propose the realization of two types of wearable
wireless sensing systems based on MEMS
accelerometers and gyroscopes, able to recognize in
real time specific kinetic features associated to
motion disorders typical of (but not limited to) the PD
and eventually give an auditory stimulation to the
patient to release the involuntary block. They have
been designed to be used at home or outdoor, during
the daily patient life. One system has the sensor in a
headset and uses an artificial neural network (ANN)
for the recognition of the motion features as regular
steps, short steps, gait blocks, trunk oscillations.
Another headset system recently proposed in
152
Kita, A., Lorenzi, P., Romano, G., Rao, R., Parisi, R., Suppa, A., Bologna, M., Berardelli, A. and Irrera, F.
Smart Sensing System for the Detection of Specific Human Motion Symptoms of the Parkinson’s Disease.
DOI: 10.5220/0005666801520159
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 152-159
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
literature (L. Atallah et al., 2014) uses only
accelerometers just to detect the gait asymmetries
without making any recognition of specific gait
features (which is the topic of our system). The other
system proposed here has two sensors on the shins
and uses a time-based algorithm for the recognition.
Compared to other systems, the headset has the
advantage that it is composed by a single sensor
integrated in the headphone. This makes the system
compact and energy efficient since no wired/wireless
connection is required to give the audio-feedback. On
the other hand, the headset has the disadvantage that
the neck joint mixes signals from the amount of
postural problems and irregular movements typical of
the Parkinson disease, which makes the detected
traces extremely "noisy" and confused (as
experimentally proved).
The second system proposed here requires an
additional device for the audio feedback, but the two
sensors on the shins guarantee the best performance
presented in literature to date in terms of sensitivity,
specificity, precision and accuracy. It has been tested
on a population of PD patients with excellent results.
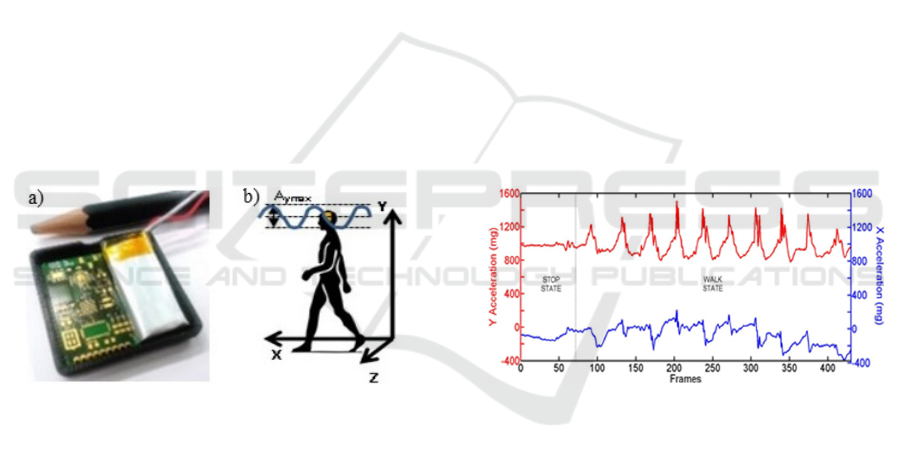
The board used in the two systems is a prototype
called neMEMSi (D.Comotti et al., 2014) whose size
is 25x30x4 mm3 (with battery, see Fig. 1a).
Figure 1: a) A picture of the NeMEMSi board. b) sketch of
the reference framework of the headset sensor.
The sensor unit LSM9DS0 integrates a ±16 g (g-
force) 3D accelerometer, a ±12 Gauss 3D
magnetometer and a ±2000 dps 3D gyroscope.
Bluetooth communication is supported. The board
integrates an ultralow-power 32 bit microcontroller
(MCU) by STMicroelectronics (STM32L1) with 33.3
DMIPS peak computation capability and very low
power consumption (down to 233 uA/MHz), Flash
memory 256 KB, SRAM 16 KB, EEPROM 4 KB.
Thanks to the Cortex™ M3 architecture and the 32
MHz clock frequency, this MCU is optimized for
advanced and low-power embedded computations.
Actually, until now we performed the measurements
on patients using an external station (a pc) for the
calculations, since the porting on board requires
disclosure of the MCU firmware. However, we are
fully confident that the excellent capabilities of the
MCU guarantee the same system performance since
they are redundant respect to the system
requirements. In fact, the same algorithms have been
already implemented in an Arduino platform (16 bit
MCU ATmega 328P, Flash memory 32KB, SRAM
2KB, EEPROM 1KB, Clock Speed 16MHz, MIPS
16) which is largely less performing of the STM32L1.
2 THE HEADSET SENSING
SYSTEM
2.1 The Soft Operation with an
Artificial Neural Network
This system is composed of a single sensor inserted
in a headset. The reference framework is depicted in
Fig. 1b. The y-axis represents the vertical direction,
the x-axis represents the direction of the walk. The
acceleration along the x direction (Ax, blue) and
along the y direction (Ay, red) in the two states are
drawn in Fig.2. During the walk, the two
accelerations have an oscillatory behaviour, in the
stop state Ay is around 10 m/s
2
and Ax is around 0.
Figure 2: Typical curves of raw data of Ay (upper curve)
and Ax (lower curve) during a regular walk and in the stop
state.
In the case of Fig.2, the person was first in a stop
state, then he started walking and made 10 steps. In
the walk state, 10 peaks of acceleration can be clearly
distinguished. We need to implement an algorithm
able to recognize the movement disorders typical of
PD: block, the regular steps, the irregular and short
steps, the trunk oscillations. Hereafter, the results
obtained with an artificial neural network (ANN) will
be discussed, since other algorithms revealed less
satisfactorily. We used an ANN with two layers (the
hidden and the output layer). The network consists of
10 neurons, with a sigmoid weight function,
connected in a feedforward topology. We used the
80% of the data for the training with a scaled
conjugate gradient backpropagation algorithm
Smart Sensing System for the Detection of Specific Human Motion Symptoms of the Parkinson’s Disease
153
already implemented in Matlab (C.M. Bishop et al.,
1995), (T. Chau, 2001). The remaining 20% of the
data was used to validate the algorithm. The cross
entropy is chosen as performance function (D. Kline
and V. Berardi, 2005). Ten epochs are sufficient to
train the ANN in any studied case (discussed in the
following), which indicates that the algorithm is very
light and fast.
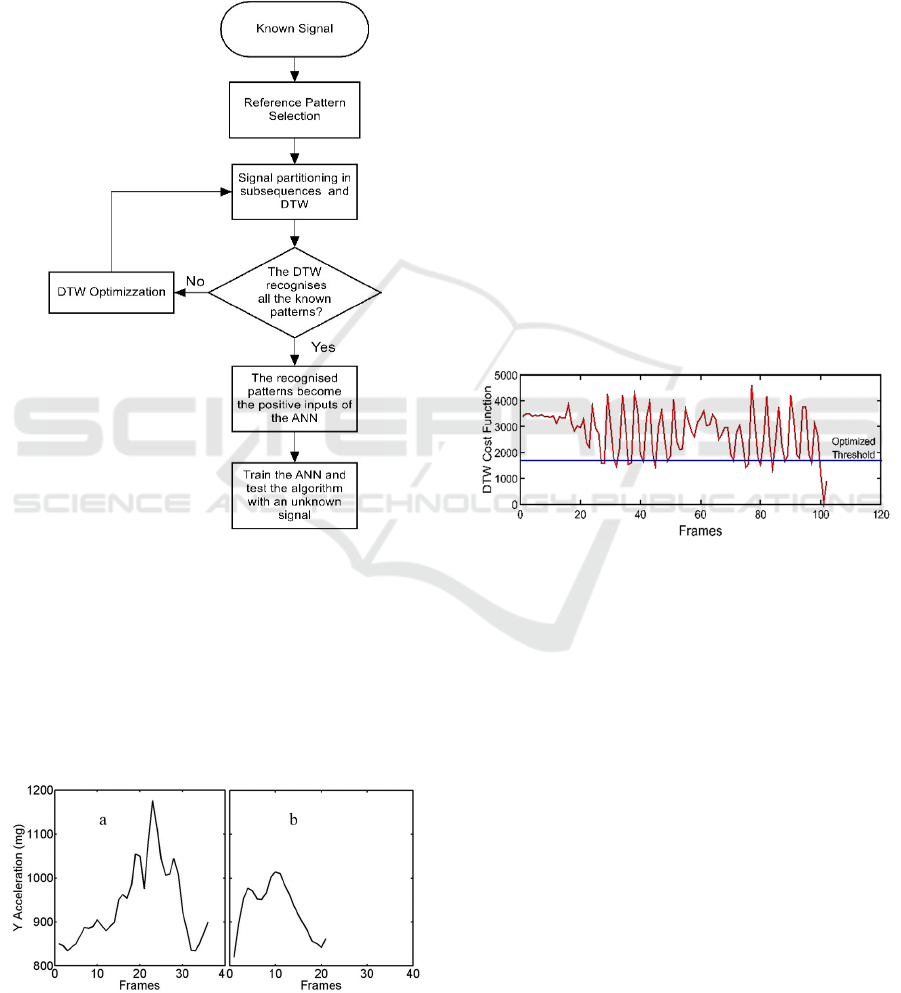
Figure 3: Flow diagram of the DTW-ANN training
procedure.
Training: The flow diagram of training is
reported in Fig.3. First of all, we choose the Ay signal
containing a known number of reference patterns with
a known size relative to steps, and we pick out a
reference pattern from it. An example of a step
reference pattern is shown in Fig.4a (selected in
region II of Fig.2).
Figure 4: (a) Reference pattern associated to a regular step.
(b) Reference pattern associated to a short step.
Apart from the amplitude, the reference pattern is
characterized by the size (number of frames) related
to the step time. The known signal is partitioned in
sub-sequences having the same size of the reference
pattern and the reference pattern is compared with the
sub-sequences. To improve flexibility, the Dynamic
Time Warping (DTW) technique is used, since it
allows comparing similar patterns rather than just one
specific pattern in the time subsequence (K.Wang et
al., 1997). DTW is a nonlinear time normalization
technique based on dynamic programming. Given
two time series of different duration, a cost function
can be calculated (E. Keogh and C. A.
Ratanamahatana, 2005). A threshold of the cost
function is set, which determines the degree of
similarity between the reference signal and the
specific subsequence. An example of the cost
function of the DTW is shown in Fig.5. When the
DTW recognizes the reference pattern in a sub-
sequence then the corresponding ANN input is
positive. On the contrary, if the known steps are not
all recognized, the size of the reference pattern and/or
the threshold of the cost function are changed (DTW
optimization) and the DTW is run again.
Figure 5: Cost function of the DTW and optimized
threshold.
2.2 Experimental Results
The ANN is now tested using unknown signals. First,
we monitored four young persons (all male) with
temporary orthopedics problems in deambulation
(defined “healthy”, in comparison with PD patients)
who made the following exercise: stop, walk a few
steps, turning, walk back, stop. The tests regarded the
detection of regular steps, the irregular gait with step
shortening (during turning), the trunk fluctuations. At
a second stage, we monitored PD patients who made
exactly the same exercise.
2.2.1 First Test on Healthy Persons: Regular
Steps and Block
The raw Ay signal of an unknown walk is plotted in
Fig.6 (upper red curve). Four intervals can be
distinguished: interval I is intuitively associated to a
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
154
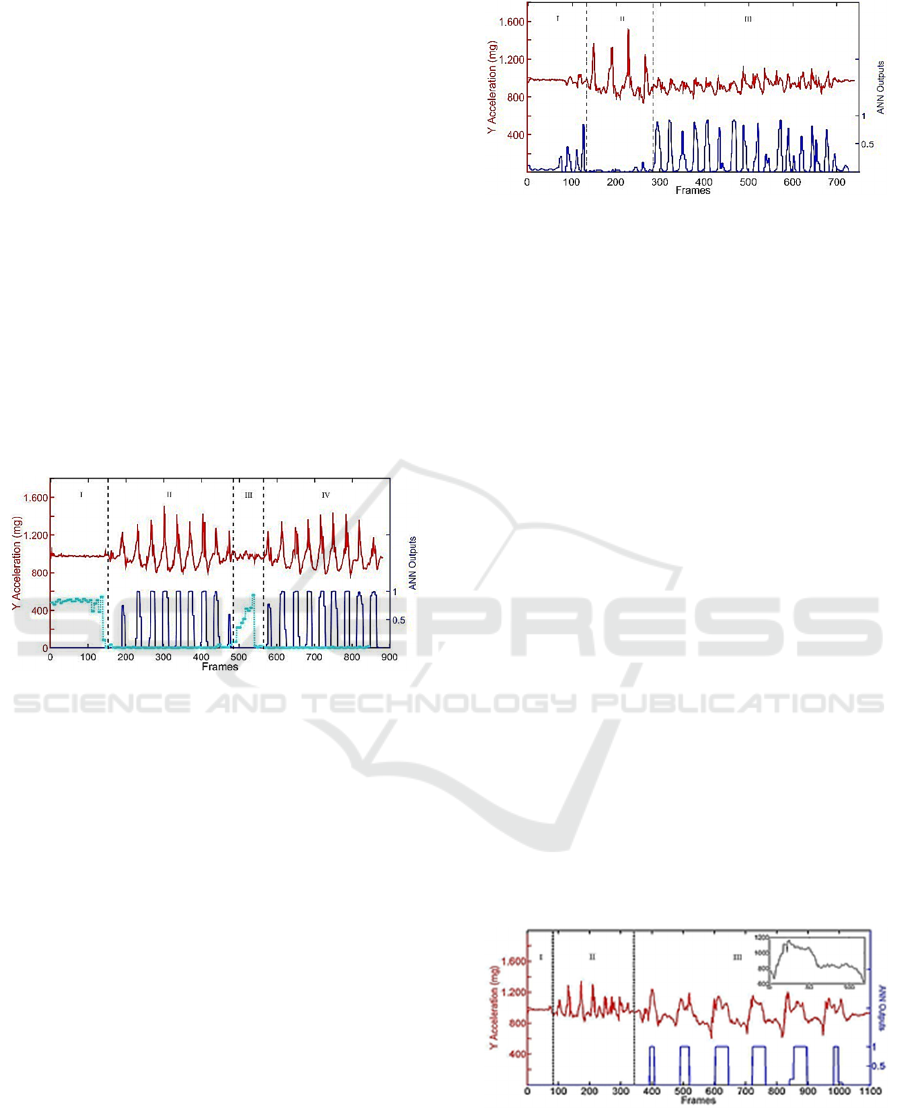
stop state (the Ay value keeps constant at 1000 mg),
intervals II and IV are clearly associated to a periodic
movement, interval III refers to an irregular gait with
short steps (while turning). The ANN was trained to
recognize the stop state. The reference signal in this
case was selected in interval I (it was an almost
straight line, not shown for brevity) and the result is
the lower dotted (ciano) curve. As expected, the ANN
output is 1 in the I interval, is 0 during intervals II and
III and assumes values between 0 and 1in the IV state,
as whether short steps were present. The presence of
regular steps was investigated using the reference
pattern of Fig.4a. The outputs of the ANN in this case
are shown in Fig.6 with the lower (blue) curve (ANN
out-puts close to 1). As one can see, nine steps were
recognized in region II and nine steps in region IV.
No regular steps were identified in region III. We can
conclude that interval III was recognized as a not
walk state and a not stop state. The irregular steps
need further investigation, and are the next focus.
Figure 6: Raw Ay signal of the first unknown test signal
(upper red curve) composed by stop state and walk state.
ANN output associated to stop (ciano) and to walk (blue).
2.2.2 Second Test on Healthy Persons: Short
Steps
The second unknown Ay signal is shown in Fig.7
(upper red curve). In this case, the exercise was
focused on the step shortening. Here, the ANN had to
recognize short steps and distinguish them from
regular ones. Fig.4a, outlines different shapes,
amplitudes, sizes). Therefore, in this experiment the
ANN was trained using a reference pattern selected in
region III. The new reference pattern is displayed in
Fig.4b with arbitrary origin. The ANN outputs are
shown in Fig.7. Although steps were irregular and
featured variable length, the ANN recognized the
short steps in interval III, where just one of the fifteen
was regarded as uncertain (step # 10). Furthermore, a
couple of irregular steps were also detected, when
passing from region I to region II and from region II
to region III.
Figure 7: Raw Ay signal of the second unknown test signal
(upper curve) composed by stop, walk state and irregular
short steps. ANN output associated to the irregular short
steps (lower curve).
2.2.3 Third Test on Healthy Persons: Trunk
Oscillations
In this test, the ANN had to recognize trunk
fluctuations in the x-y plane (referring to Fig.1b). In
this experiment, legs were motionless and only the
trunk oscillated pivoting on the pelvis. This situations
is of particular interest because during a freezing of
gait PD patients feel that their feet are stuck to the
ground and they try repeatedly to make a step
thrusting out and overbalancing. This is clearly
associated to an increased risk of fall. In this case, the
fact that the sensor is positioned on the head
guarantees the maxi-mum sensitivity to the
movement. In this experiment, the angle respect to the
vertical axis varied in the range ±20 degrees. Again,
the Ay raw signal was analysed and the curve is
shown in Fig.8 (upper red curve). As expected, the
trunk oscillations are very well characterized (region
III). Regions I and II are associated, respectively, to a
stop state and a walk state. The ANN was trained to
recognize trunk fluctuations using a reference pattern
selected in region III. It is shown in the inset. The
ANN outputs are displayed in Fig.8 (lower blue
curve). Recognition was excellent and all the trunk
oscillations yielded ANN = 1.
Figure 8: Raw Ay signal of the third unknown test signal
(upper curve) composed by stop state, walk state and trunk
oscillations. ANN output associated to the trunk oscillations
(lower curve).
Smart Sensing System for the Detection of Specific Human Motion Symptoms of the Parkinson’s Disease
155
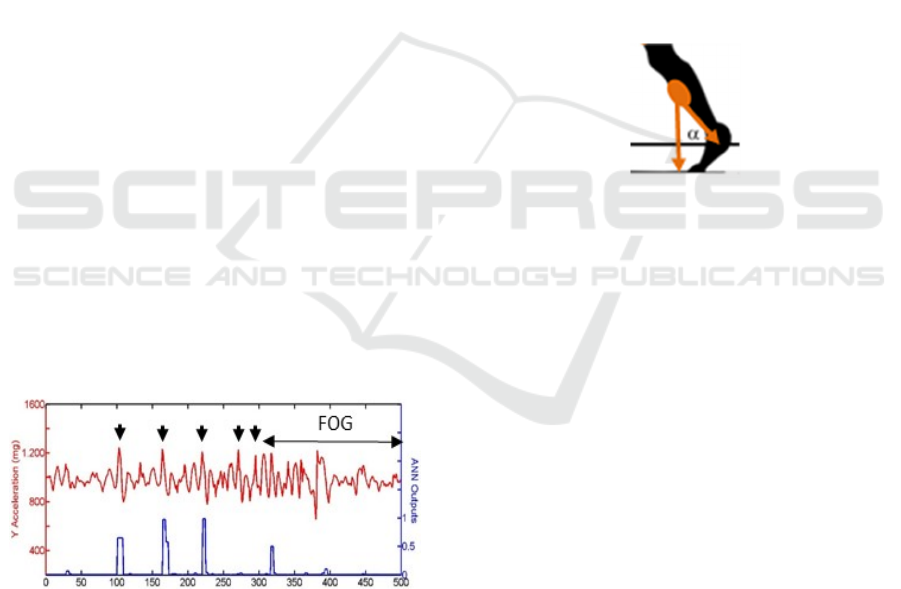
2.2.4 Test on PD Patients
All the results of the tests discussed above revealed
that the headset system recognizes successfully the
gait features and the trunk (and head) movements of
healthy persons with temporary orthopedic problems.
Then, we started monitoring PD patients, but we
limited to just a few (male, over 70) since results were
not satisfactorily. Patients were asked to make the
exercise described before. Tests were registered by a
camera and supervised by doctors in order to establish
the exact starting and ending time of the eventual
FOG event. As an example, Fig.9 reports results
relative to one of the PD patients experiencing a FOG
during the test. The patient made four regular steps
and a fifth short step, very close to the previous one.
This fifth step defines an incipient FOG (pre-freezing
state). Then the FOG event occurred, during which
the patient made some irregular movements of the
whole body, without making steps. The five steps are
outlined with arrows on the Ay curve sketched in the
figure. Looking at the ANN output, the first three
steps are correctly detected, step 4 and step 5 are false
negative, whereas a false positive is present during
the FOG state, probably confusing a large movement
of the body with a step. We can conclude that the
headset is not similarly effective on PD patients as on
healthy persons. This is due to the fact that PD
patients feature a great variety of postural problems
and irregular movements in many sections of the
body, all mixed together. The headset device suffers
from the presence of a joint (the neck) which can mix
(or hide part of) the signals, making the information
extremely "noisy" and confused, thus introducing
false positive and false negative outputs.
Figure 9: Raw Ay signal of the test signal taken on a PD
patient (upper curve) composed by four regular steps, one
short step close to the forth one, a FOG during which the
body makes oscillations. The ANN reveals false negative
and false positive outputs.
3 THE SENSING SYSTEM ON
THE SHINS
In order to monitor movement disorders specifically
in PD patients we designed another system with
sensors positioned on the shins. In this case, the
recognition algorithm is based on a time domain
analysis of the sensor signals. The raw signals of
accelerometers and gyroscopes are fused together
through the attitude and heading reference system
(AHRS) and the Madgwick's algorithm (β=0.15)
(Madgwick et al., 2011). The data reading frequency
from the sensor is 60Hz, which allows a correct
sampling of the signal during FOG events since the
relevant spectrum of FOG is 3 – 10 Hz. A quaternion
based representation of the limb orientation and
position is calculated. The angles α
right
and α
left
between the vertical axis and the right/left shin are
sketched in Fig.10.
Figure 10: Angle between the vertical axis and the shin.
The angular velocities ω
right
, ω
left
obtained after
angle derivation are used as the input for the FOG
detection. A new algorithm was developed which
calculates the low-pass of the angular velocities:
k
ri
g
h
t
= lowpass(|ω
ri
g
h
t
|) (1)
k
Lef
t
= lowpass(|ω
Lef
t
|) (2)
and introduces an index K = k
right
+ k
left
. The
algorithm in eqs.(1) and (2) is an improvement of a
very recent algorithm proposed in literature (Y. Kwon
et al., 2014), which uses the root mean square of the
accelerometer signal of a single sensor and does not
perform fusion with the gyroscope signal. Actually,
thanks to the fusion of gyroscope and accelerometer
signals, our algorithm allows to achieve a higher
precision. This is paid in terms of the number of
calculations, but the low pass filtering in the
equations above needs a lower number and a lower
rate of accesses to the microcontroller memory,
respect to the root mean square method. As a matter
of fact, we tested both algorithms and the proposed
one exhibited better performance in terms of
precision, with comparable calculation time.
A population of sixteen patients of different age
and sex, at different stages of the disease was asked
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
156
to wear the two sensors and make an exercise several
times. The population is described in Table I. The
exercise was: walking some steps, passing through an
open door, turning and going back.
Table 1: Sex, age, disease stage of monitored patients.
male/female
under/over 65 early/advanced
9/7 5/11 6/10
FOG events occurred frequently during the
exercises, especially when passing through the open
door and during turning. In order to classify properly
the states, a preliminary calibration of the system was
performed. To this aim, the whole exercise was
filmed with a camera and the sensor signals were
recorded. The films were studied by doctors, who
indicated the exact timing of the freezing events.
Then, the calculated K curves were compared with
the clinical observations by the doctors. This allowed
defining three threshold values of K (T1-3) which
classify the four states: regular gait (K>T3), pre-post
freezing-state (T3>K >T2), involuntary freezing state
(T2>K>T1) and voluntary rest state (K<T1). It is
worth noticing that the values of T1-3 are the same
for all the patients. From clinical side, distinguishing
the involuntary freezing state from the rest state is
crucial, and, fortunately, it relatively simple using
inertial sensors since in the involuntary freezing state
the muscle activity is always present and gives rise to
lots of small movements which are clearly detected
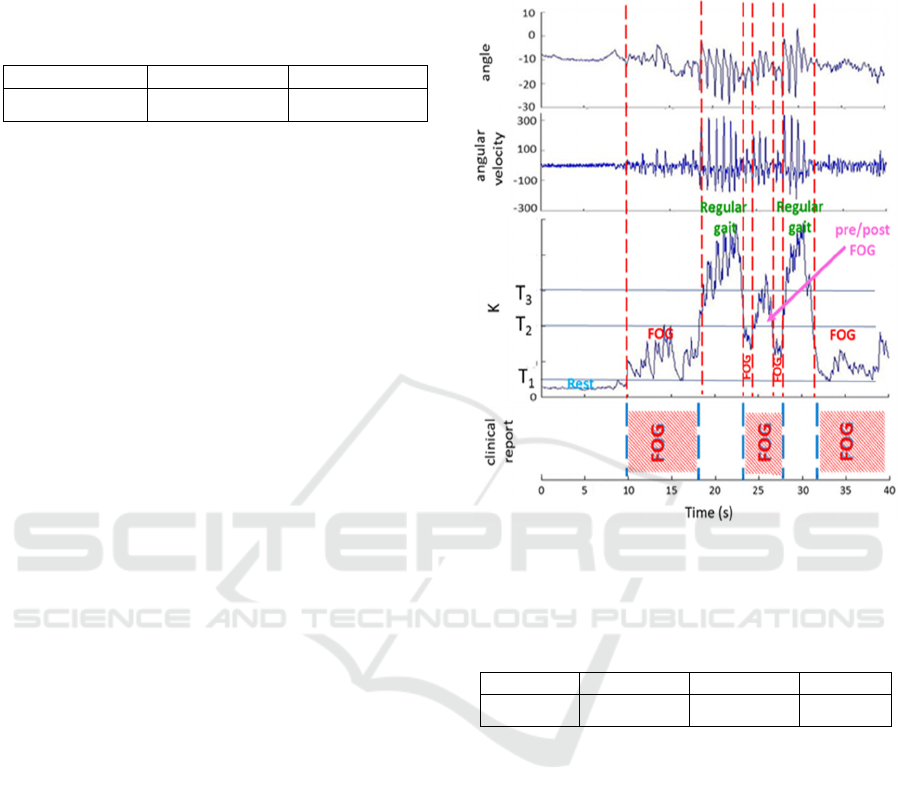
by the sensors. An example of α, ω and K is shown
in the diagrams of Fig.11. Clinical report by the
doctor about the exact FOG timing is sketched in the
bottom diagram. The comparison between the K
curve and the clinical reports allowed defining the T
thresholds and the four classified states. In the
example of Fig.11, a few FOG and pre-FOG events
were identified by both doctors and the system. In one
case (time=23-28s), the system distinguished
between pre-FOG and FOG states, whereas doctors
reported just a FOG in the whole time interval. Values
of T
1-3
remained the same along all the measurements.
Subsequent cross checks outlined an excellent
agreement between the doctors reports and the
automatic recognition of FOG performed by our
system. An extremely low number of errors (false
positive or false negative) were found. The particular
algorithm implemented allowed to get the best
performance published to date in terms of sensitivity,
precision, accuracy and specificity. The average
results on about two hours recording time and sixteen
patients are shown in Table 2. As a comparison with
the state of art, another system using inertial sensors
positioned on the ankles featured a sensitivity of 77
% and a specificity of 86.5 % (S. Mazilu et al., 2013).
This result was obtained on a population of fourteen
PD patients.
Figure 11: An example of angle and angular velocity
measured by the sensor on the shin during the exercise. The
calculate K index is also displayed. The bottom diagram
reports the clinical observation of the FOG events timing.
Table 2: Performance of the system.
Sensitivity Specificity Precision
Accuracy
94.5% 96.7% 93.8% 95.6%
4 CONCLUSIONS
In this paper we proposed the realization of two
wearable wireless sensing systems based on silicon
integrated micro-electro-mechanical inertial sensors
able to recognize in real time specific kinetic features
associated to human motion disorders. The system is
designed specifically for the Parkinson’s disease and
gives an auditory stimulation to the patient to release
block states in the freezing of gait. One system has
the sensors in a headset, while the other one has
sensors on the shins. They can be used at home or
outdoor, during the daily activity of the patient. The
hardware used for the two solutions is the same and
uses the same integrated sensors. On the contrary,
different algorithms were implemented in the two
cases, accounting for the distinct peculiarities of the
Smart Sensing System for the Detection of Specific Human Motion Symptoms of the Parkinson’s Disease
157

two solutions. In the case of the headset, a number of
different algorithms were used in order to improve
recognition of the gait features. In the paper, we
discussed results regarding the recognition of the
block state, regular and irregular steps, trunk
oscillations obtained with an artificial neural network.
For the system with sensors on the shins we used an
algorithm filtering and processing in real time the
angular velocities of the two legs. This gives excellent
recognition of the irregularities of each step and
detects even barely perceptible tremors in all the
monitored PD patients, allowing distinguishing
doubtless between the voluntary stop state and the
involuntary block due to the FOG. Further
optimization and simplification of the detection
algorithm can be achieved by better manipulating the
quaternions representation of the limbs.
The headset has advantages in terms emphasized
sensitivity to trunk oscillations, easy wearability and
direct auditory feedback. This implies an excellent
detection of specific typologies of motion disorders,
and makes the system compact and energy efficient
since gives the audio-feedback without any
wired/wireless connection. Unfortunately, PD
patients feature a great variety of postural problems
and irregular movements in all the sections of the
body, and the system suffers from the presence of a
joint (the neck) which can mix (or hide part of) the
signals, making the information extremely "noisy"
and confused, thus introducing false positive and
false negative outputs. For this reason, the headset
can be better employed for other types of motion
disorders, as in the case of temporary orthopedics
ones.
The other device requires an additional device in
the ear for the audio-feedback, but guarantees the best
performances presented in literature to date in terms
of sensitivity, specificity, precision and accuracy in
the detection of the FOG events. The system was
validated on a population of sixteen patients of
different age, sex and stage of the disease.
ACKNOWLEDGEMENTS
Authors thank the patients for credit and forbearance,
and STMicroelectronics for providing the NeMEMSi
boards.
REFERENCES
Arias, P. and Cudeiro, J. "Effect of Rhythmic Auditory
Stimulation on Gait in Parkinsonian Patients with and
without Freezing of Gait" PLoS ONE. Vol.5 (2010).
Atallah, L., et al. "Gait asymmetry detection in older adults
using a light ear-worn sensor." Physiological
measurement 35.5 (2014): N29.
Bachlin, M. et al. "A wearable system to assist walking of
Parkinson s disease patients" Methods Inf Med, vol.49,
pp.88–95, 2010.
Bishop, C.M. et al. “Neural networks for pattern
recognition. Clarendon, Oxford (1995).
Bloem, B.R. et al. "Falls and freezing of gait in Parkinson's
disease: a review of two interconnected, episodic
phenomena." Movement Disorders 19.8 (2004): 871-
884.
Chau T. “A review of analytical techniques for gait data”
Part 2: neural network and wavelet methods. Gait &
Posture. Vol.13 (2001) 102–120.
Cola, G. et al. "An On-Node Processing Approach for
Anomaly Detection in Gait." Sensors Journal, IEEE ,
vol.15, no.11, pp.6640-6649, Nov. (2015).
Comotti, D. et al. "neMEMSi: One step forward in wireless
attitude and heading reference systems" Inertial Sensors
and Systems (ISISS), Intern. Symposium on 1–4
(2014).
Keogh E. and Ratanamahatana C. A.: Exact indexing of
dynamic time warping. Knowledge and information
systems. Vol.7 (2005) 358–386.
Kline D. M. and Berardi V. L.: Revisiting squared-error and
cross-entropy functions for training neural network
classifiers. Neural Computing & Applications. Vol.14
(2005) 310–318.
Kwon, Yuri, et al. "A practical method for the detection of
freezing of gait in patients with Parkinson’s disease."
Clinical interventions in aging 9 (2014): 1709.
Lorenzi, P. et al. “Wearable Wireless Inertial Sensors for
Long-Time Monitoring of Specific Motor Symptoms in
Parkinson’s Disease” BIODEVICES 2015 - 8th Int.
Conference on Biomedical Electronics and Devices,
Proceedings; Part of 7th International Joint Conference
on Biomedical Engineering Systems and Technologies,
BIOSTEC 2015, Lisbon (P) pp.168-173.
Madgwick, S.O.H et al. "Estimation of IMU and MARG
orientation using a gradient descent algorithm", IEEE
International Conference on Rehabilitation Robotics ,
Rehab Week Zurich, ETH Zurich Science City,
Switzerland, 2011.
Mazilu, S. et al. "Feature learning for detection and
prediction of freezing of gait in Parkinson’s disease."
Machine Learning and Data Mining in Pattern
Recognition. Springer Berlin Heidelberg, 2013. 144-
158.
Mazilu, S. et al. "GaitAssist: A Daily-life Support and
Training System for Parkinson's Disease Patients with
Freezing of Gait", Proceedings of the SIGCHI
Conference on Human Factors in Computing Systems
2531–2540 (2014).
Moore, S. et al. "Autonomous identification of freezing of
gait in Parkinson's disease from lower-body segmental
accelerometry" Journal of NeuroEngineering and
Rehabilitation, vol.10, pp.19, 2013.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
158

Nieuwboer, A. et al. "Abnormalities of the spatiotemporal
characteristics of gait at the onset of freezing in
Parkinson's disease." Movement Disorders 16.6 (2001):
1066-1075.
Sijobert, B. et al. "IMU Based Detection of Freezing of Gait
and Festination in Parkinson's Disease" IFESS
MALAYSIA, 2014.
Spildooren, J. et al "Freezing of gait in Parkinson's disease:
the impact of dualtasking and turning" Movement
Disorders25.15 (2010)2563-70.
Wang, Kongming, et al. Alignment of curves by dynamic
time warping. The Annals of Statistics, (1997), 25.3:
1251-1276.
Smart Sensing System for the Detection of Specific Human Motion Symptoms of the Parkinson’s Disease
159
