
Experimental Analysis and Modeling of NO
x
Emissions in
Compression Ignition Engines Fueled with Blends of Diesel and Palm
Oil Biodiesel
Adriana Patricia Villegas Quiceno
1
, Ramón Fernando Colmenares Quintero
1
, Simona Silvia Merola
2
,
Adrian Irimescu
2
and Gerardo Valentino
2
1
Research Group TERMOMEC, Universidad Cooperativa de Colombia, Medellín, Colombia
2
Istituto Motori CNR, Napoli, Italy
Keywords: Compression Ignition Engine, Modeling, NO
x
Emissions, Blends, Diesel, Biodiesel.
Abstract: In this work, theoretical and experimental studies about the effects of the blends of diesel and palm oil
biodiesel on NO
x
formation in compression-ignition engines were developed. Experiments were conducted
using commercial diesel, palm oil biodiesel and blends at 5% (B5), 20% (B20) and 50% (B50) as fuels. A
phenomenological semi-empirical model that uses the information obtained from thermodynamic diagnostics
was used for determining the theoretical NO
x
formation. The model shows the high sensitivity of NO
x
formation to the temperature and the operating conditions. Effects associated to the operating conditions of
the engine were evaluated and the results indicate that high engine loads and low speeds lead to the NO
x
formation. However, it was not possible to determine with precision, the effect of the type of fuel, because of
the high sensitivity of the NO
x
formation with respect to the operating conditions of the engine.
1 INTRODUCTION
Nowadays, biodiesel has received considerable
attention given its potential use as a substitute for
petroleum diesel. In general terms, the current
technology is easily adapted for the use of such a fuel,
since its implementation does not require significant
changes in the control strategy of diesel engines.
Biodiesel is a renewable fuel that reduces greenhouse
emissions, such as particulate matter, carbon
monoxide, and total hydrocarbons among others
(Agudelo et al., 2010) (Sun et al., 2010).
However, the effect of the biodiesel and its blends
with diesel fuel on NO
x
formation is still a topic of
discussion in the literature. (Lapuerta et al., 2008) and
(Sun et al., 2010) classify the literature regarding the
effect of the biofuels on NO
x
production in five
groups: group 1: NOx increase, group 2: NO
x
increase
under certain operating conditions and blends, group
3: little or no difference, group 4: NO
x
decrease and
group 5: uncertain or no comment. The increase in
NO
x
emissions generates serious problems for public
health and the environment as these chemical species
contribute to the production of ozone. High levels of
ozone at low altitude cause respiratory diseases and
damage to the vegetation among others. On the other
hand, nitric oxides increase the level of acid rain and
contribute to the production of photochemical smog
(Turns, 1996). Also, NO
x
affects the development of
the biofuels industry due to the stringent restrictions
for these emissions in American and European
regulations. Several factors could increase or
decrease the NO
x
formation in blends of diesel and
biodiesel: adiabatic flame temperature, ignition delay
(Eckerle et al., 2009) and injection timing, as well as
fuel chemistry (Ban-Weiss et al., 2007). These factors
are determinant in the process of NO
x
formation and
should be analyzed as an overview rather than as
individual effects (Ban-Weiss et al., 2007). Adiabatic
flame temperature is affected by the fuel chemistry.
Increased aromatic content produces higher flame
temperatures that can promote NO
x
formation
through the thermal mechanism. This effect is most
significant for modes of combustion dominated by
diffusion, which mainly occur a high engine load
operation (Eckerle et al., 2009).
Ignition delay is defined as the time between the
start of injection and start of combustion; this
parameter is generally shorter for biodiesel compared
to petroleum diesel (Sun et al., 2010). The cetane
Quiceno, A., Quintero, R., Merola, S., Irimescu, A. and Valentino, G.
Experimental Analysis and Modeling of NO
x
Emissions in Compression Ignition Engines Fueled with Blends of Diesel and Palm Oil Biodiesel.
In Proceedings of the International Conference on Vehicle Technology and Intelligent Transport Systems (VEHITS 2016), pages 245-252
ISBN: 978-989-758-185-4
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
245

number is an indicator of the ignition delay time;
higher values correspond to shorter ignition delay
times (Ban-Weiss et al., 2007). It has been reported
that the sensitivity of NO
x
to changes in Cetane
number is higher at low load than at high load
(Lapuerta et al., 2008) which could reduce NO
x
emissions at low load. Advanced injection timing
could produce higher NO
x
emissions as the
combustion stars earlier, and thus the residence time
of the burning mixture in the cylinder is increased. An
advance in injection timing for biodiesel with respect
to petroleum diesel is caused by its higher bulk
modulus of compressibility for the biodiesel (Ban-
Weiss et al., 2007).
In this paper, a theoretical and experimental
analysis of the NO
x
production using diesel and palm
oil biodiesel blends is discussed The mechanism of
formation of NO
x
through the thermal mechanism is
presented and a semi-empirical model is applied to
determine the NO
x
formation in the combustion
diffusion phase.
2 METHODOLOGY
2.1 Modeling of NO
x
Emissions in
Diesel Engines
During combustion of fuels that do not contain
nitrogen in their structure, nitrogen monoxide (NO) is
formed from three mechanism: the thermal
(Zeldovich), the prompt (Fenimore) and fuel-bound
nitrogen (Sun et al., 2010). The thermal mechanism is
presented during high temperatures in the combustion
chamber when the reaction between the oxygen and
the nitrogen is carried out (Miller and Bowman,
1989). The thermal mechanism represents the most
important source in the NO
x
formation (Ban-Weiss et
al., 2007) and it is represented by three reactions: O +
N
2
↔ NO + N, O + O
2
↔ NO + O and O + OH ↔ NO
+ H (Sun et al., 2010). Some simplifications have
been applied to these equations in order to obtain the
general dynamic expression for NO
x
production
presented as d[NO] / dt = 6 · 10
16
/ T
0.5
exp(-69.09 /
T) · [O
2,eq
]
0.5
· [N
2,eq
] (Sun et al., 2010) (Fernando et
al., 2006) (Park et al., 2013). The first assumption is
that the nitrogen chemistry can be de-coupled from
combustion reactions due to the reactions rate of non-
nitrogenated species generally occur much faster than
that of the nitrogen chemistry, The second approach
implies that the concentrations of O, O
2
, OH, H and
N
2
can be approximated to the equilibrium
temperature. Finally, it is assumed that the radical N
is in its steady state. An approach for solving the
equation for calculating d[NO] consists in applying a
calculation scheme that takes into account the main
kinetics aspects for the formation and destruction of
NOx in the combustion chamber, which is linked to
the engine operating conditions. The main variables
include the instant pressure in the combustion
chamber, the fuel mass burned rate (FMB), the
adiabatic flame temperature (T
ad
) and the heat release
rate (HHR) which are obtained from thermodynamics
diagnostics (Timoney et al., 2005) (Park et al., 2013)
(Guardiola et al., 2011).
Based on the model developed by (Dec, 1997), it
is possible to confirm that the premixed combustion
phase does not show NO
x
formation as the higher
equivalent ratio reduces the amount of available
oxygen. This situation implies that the most important
processes for NO
x
formation occur in the flame front
during the combustion by diffusion.
Bearing this in mind, the theoretical NO
x
production is determined in the combustion diffusion
phase and the adiabatic flame temperature is the
characteristic temperature. The combustion diffusion
phase is taken from the values of heat release rate
curves between the start of combustion (SOC) and the
end of diffusion phase (EOD) (Timoney et al., 2005).
The approximated values of adiabatic flame
temperature for the biodiesel and their blends with
diesel are taken from (Glaude et al., 2010). The
equilibrium concentrations are estimated under
stoichiometric conditions and constant pressure. The
NASA simulation program “Chemical Equilibrium
Program” was used for calculating the equilibrium
concentrations.
Taking into account all the above considerations
(Timoney et al., 2005), a final expression for the
determination of NOx in each operating point is as
follows: e
NOx
= k Σ
SOC
EOC
T
ad
0.5
·ΔFMB · n
-1
· P
-1
·
exp(-0.69 / T
ad
) · [O
2,eq
]
0.5
· [N
2,eq
] · ΔCAD, where
constant k = Ru 6 · 10
16
· (1 + SFAR) / (6 NSTEP M
W
b
), with FMB calculated based on the rate of heat
release, with the lower heating value of each fuel
NSTEP and ΔCAD corresponding to the angular
resolution.
2.2 Experimental Setup
Test were carried out in a bench-mounted and
instrumented automotive diesel engine (Table 1). The
engine was coupled to a 230 kW Eddy current
dynamometer. Air and fuel consumptions were
measured with a hot-wire sensor and an electronic
balanced mass flow sensor, respectively. NO
x
were
measured with chemiluminescense analyzer. In-
cylinder combustion diagnostics was carried out
SFFEV 2016 - Special Session on Simulation of flex fuel engines and alternative biofuel vehicles
246

using a two species (air and combustion products),
single-zone model, based on the approach proposed
by (Lapuerta et al., 1999). For recording the
instantaneous in-cylinder pressure, a piezoelectric
pressure transducer installed on the glow plug and a
Kistler 5011B charge amplifier were used. The
instantaneous piston position was determined using
an angular encoder with a resolution of 1024
pulses/revolution coupled to the crankshaft at the
opposite end of the fly-wheel. The angle of start of
injection was measured with a clamp-on transducer.
Table 1: Engine specifications.
Reference ISUZU 4AJ1
Type Turbocharged, direct injection,
rotating pump
Swept volume 2499cm
3
Configuration 4 in-line cylinders
Bore x stroke 93mm x 92mm
Compression ratio 18.4
Rated Power 59kW at 4100 rpm
Maximum torque 170Nm at 2300 rpm
Tests were performed using palm oil biodiesel
(BP100), commercial grade diesel fuel and three
diesel biodiesel blends (BP5, BP20, BP50). The
properties of fuels are presented in Table 2. For each
fuel, twelve operation modes, each one characterized
by an effective torque (20, 40, 60 and 80 Nm) and
engine speed (2000, 2250 and 2500 rpm) were tested,
with NO
x
emission measurements performed in
steady state conditions.
3 RESULTS AND ANALYSIS
3.1 Experimental Analysis of NO
x
Emissions
In Table 3, the experimental results of the NO
x
measurements at different operating conditions and
blends of diesel and palm oil biodiesel are presented.
The trends in NO
x
emissions were evaluated using
statistical software. The Analysis of variance
(ANOVA) shown in Table 4 and the confidence
intervals given in Table 5 indicate that the type of
biodiesel, speed and torque affect significantly on the
NO
x
emissions.
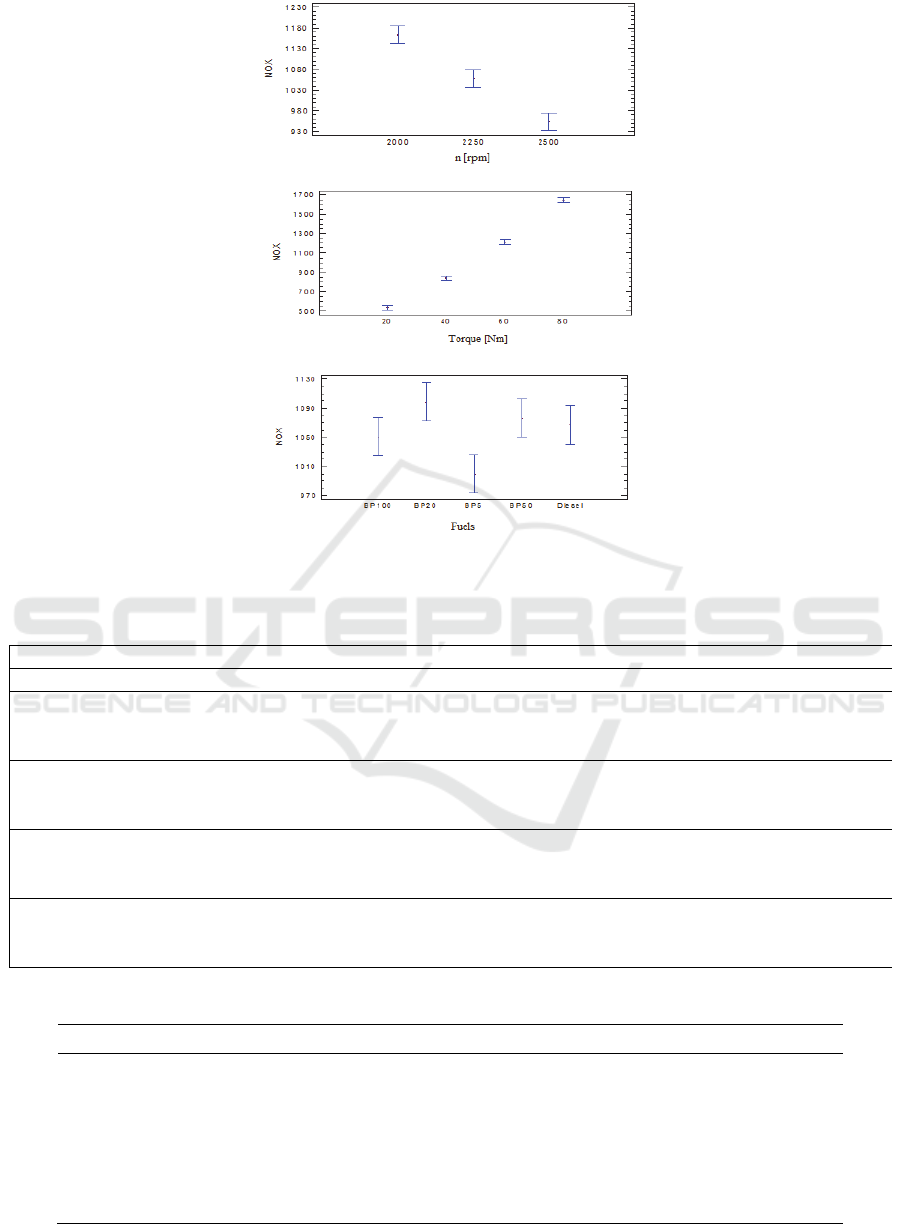
Figure 1 (a) suggests that the NO
x
formation
decreases with an increase of the engine speed for all
fuels in all torque settings. Two factors could produce
this trend, the mean temperature in the combustion
chamber and the residence time of combustion gases.
Table 6 shows the highest mean temperatures within
the combustion chamber for all operation modes and
fuel blends. Although the mean maximum
temperature increases with the engine speed at the
same load, the time of the residence of the
combustion gases decreases leading to low values of
NO
x
in the range of speeds evaluated.
It is important to highlight at this point, that in the
work of (Agudelo et al., 2010) it was observed that
the NO
x
increases with the speed of the engine in
regions near to the maximum torque of the engine (i.e.
170 Nm at speeds lower than 1750 rpm).
As it can be seen in Figure 1(b), The NO
x
formation increases as the Torque goes up. This
Table 2: Fuel properties.
Fuel Chemical Formula
(b)
Molecular
weight
(b)
[kg/kmol]
Density at
15°C [kg/m
3
]
Lower Heating
Value [kJ/kg]
(a)
Stoichiometric
air/fuel ratio
()
(c )
Diesel
.
.
.
208.2 853.4 41568 14.8
BP100
.
.
283.5 879.7 37920 12.8
BP005
.
.
.
211.1 854.7 41444 14.5
BP020
.
.
.
220.1 858.7 41075 14.2
BP050
.
.
.
237.3 866.6 40387 13.9
(a)
calculated from ultimate composition and measured gross heating value
(b)
calculated from fatty acid methyl esters profile
(c)
calculated from composition
Experimental Analysis and Modeling of NO
x
Emissions in Compression Ignition Engines Fueled with Blends of Diesel and Palm Oil
Biodiesel
247
(a)
(b)
(c)
Figure 1: Results of variance analysis for NO
x
. Means and 95% of Fisher LSD.
Table 3: NO
x
emissions at different operating conditions and blends of biofuels.
NO
x
emissions [ppm]
Torque [Nm] n [rpm] Diesel BP5 BP20 BP50 BP100
20
2000 568 601 619 602 565
2250 537 516 566 564 497
2500 468 485 491 491 486
40
2000 940 897 979 956 856
2250 854 867 862 836 805
2500 753 759 802 724 730
60
2000 1358 1159 1412 1402 1359
2250 1234 1089 1247 1228 1183
2500 1089 1079 1111 1105 1092
80
2000 1827 1508 1879 1900 1898
2250 1663 1631 1682 1646 1646
2500 1515 1409 1534 1461 1495
Table 4: Variance analysis for NO
x
emissions.
Source Sum of squares Gl Medium square F-Ratio P-Value
Principal effects
A Fuel 65783,6 4 16445,9 4,03 0,0066
B:[rpm] 442289, 2 221144, 54,19 0,0000
C:Torque [Nm] 1,03112E7 3 3,43708E6 842,22 0,0000
Residuals 204049, 50 4080,98
Total (Correction) 1,10234E7 59
SFFEV 2016 - Special Session on Simulation of flex fuel engines and alternative biofuel vehicles
248
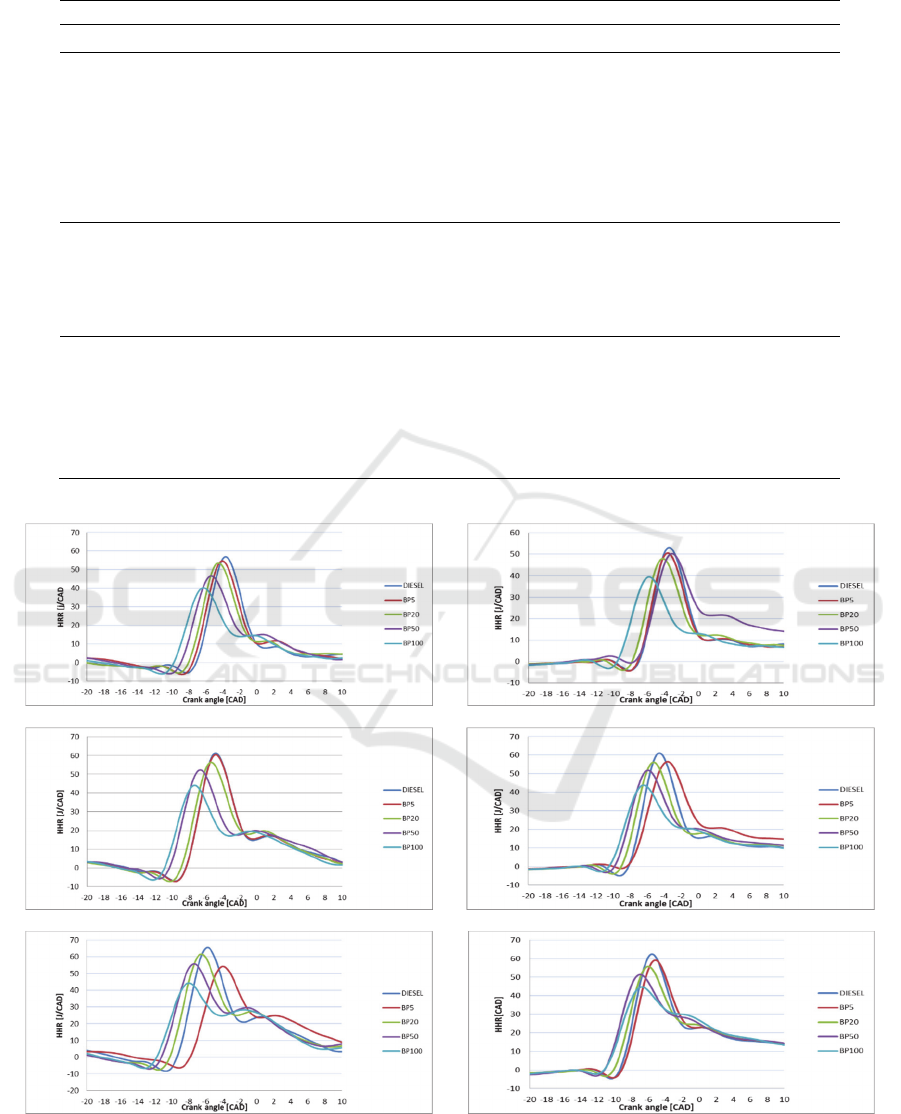
Table 5: Mean and least squares for NO
x
emissions with confidence intervals of 95%
Level Cases Means Error Est. Lower Bound Upper Bound
Means Global 60 1058.62
Fuel
BP100 12 1051.0 (1.51%) 18.4413 1013.96 1088.04
BP20 12 1098.67 (-2.86%) 18.4413 1061.63 1135.71
BP5 12 1000.0 (6.29%) 18.4413 962.959 1037.04
BP50 12 1076.25 (-0.84%) 18.4413 1039.21 1113.29
Diesel 12 1067.17 18.4413 1030.13 1104.21
n [rpm]
2000 20 1164.25 14.2846 1135.56 1192.94
2250 20 1057.65 14.2846 1028.96 1086.34
2500 20 953.95 14.2846 925.259 982.641
Torque [Nm]
20 15 537.067 16.4944 503.937 570.197
40 15 841.333 16.4944 808.203 874.463
60 15 1209.8 16.4944 1176.67 1242.93
80 15 1646.27 16.4944 1613.14 1679.4
(a) (d)
(b) (e)
(c) (f)
Figure 2: Heat release curves at low loads operating conditions: 20 Nm at (a) 2000 rpm, (b) 2250 rpm, (c) 2500 rpm, and 40
Nm at (d) 2000 rpm, (e) 2250 rpm, (f ) 2500 rpm.
Experimental Analysis and Modeling of NO
x
Emissions in Compression Ignition Engines Fueled with Blends of Diesel and Palm Oil
Biodiesel
249
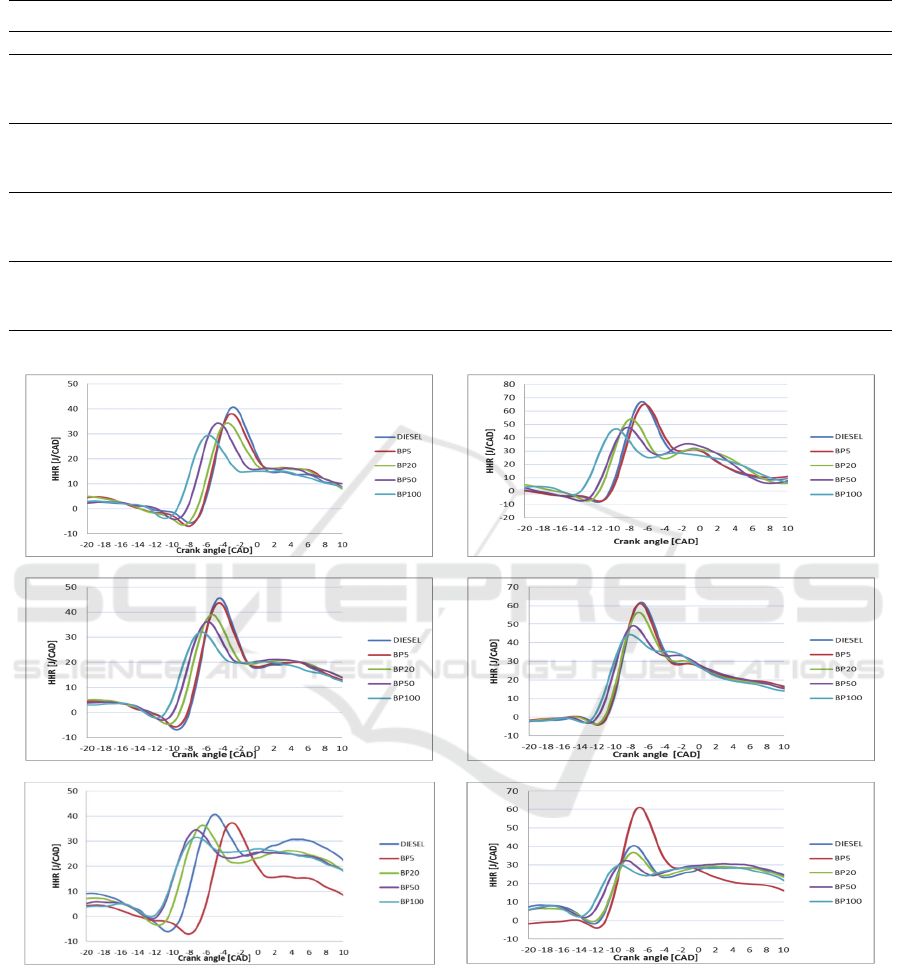
Table 6: Maximum mean temperature in the combustion chamber.
Maximum Mean Temperature [K]
Torque [Nm] n [rpm] Diesel BP5 BP20 BP50 BP100
20
2000 1277.23 1259.62 1310.14 1339.17 1293.06
2250 1390.79 1365.97 1419.92 1462.61 1413.00
2500 1482.81 1420.41 1542.48 1568.07 1513.18
40
2000 1291.93 1254.99 1313.07 1518.04 1300.15
2250 1423.00 1493.65 1442.08 1494.96 1429.33
2500 1550.60 1503.25 1562.17 1607.92 1544.56
60
2000 1334.43 1318.25 1339.76 1376.44 1316.89
2250 1481.05 1450.43 1503.72 1545.67 1487.73
2500 1659.09 1115.52 1605.96 1608.45 1621.06
80
2000 1531.19 1512.34 1576.29 1407.86 1575.24
2250 1634.31 1611.54 1607.21 1617.11 1595.88
2500 1688.41 1611.54 1673.49 1751.85 1681.67
(a) (d)
(b) (e)
(c) (f)
Figure 3: Heat release curves at high loads operating conditions: 60 Nm at (a) 2000 rpm, (b) 2250 rpm, (c) 2500 rpm, and 80
Nm at (d) 2000 rpm, (e) 2250 rpm, (f ) 2500 rpm.
behavior is due to the increase of the maximum mean
temperature in the combustion chamber as shown in
Table 6. The rise of this mean temperature causes the
NO
x
formation and high loads as presented in the heat
release rate curves given in Figures (2) and (3).
Figure 2 presents the heat release rate curves at
low engine loads. As observed in this Figure, the
combustion premixed phase is predominant for all
speeds. As the load increases, the combustion
diffusion phase is dominant as presented in Figure 3,
in which according to (Dec, 1997) the highest
emissions of NO
x
are produced.
Finally, the effect of the fuel in NO
x
formation did
not present a unique trend due to the high sensitivity
SFFEV 2016 - Special Session on Simulation of flex fuel engines and alternative biofuel vehicles
250

of the engine to the operating conditions for the
blends with palm oil biodiesel. For this reason, it is
recommended for future work to determine other
physical and chemical properties such as: viscosity,
Cetane number and bulk modulus so that the effect of
type of fuels blends with palm oil biodiesel can be
better understood.
3.2 Semi-empirical Model for NO
x
Formation
The equation developed by (Timoney et al., 2005) has
been used to establish the theoretical NO
x
formation
in the combustion diffusion phase.
Table 7: NO
x
formation.
Fuel
Experimental
NO
x
[ppm]
Theoretical
NO
x
[ppm]
% discrepancy
Diesel 1515 1390 8.25
BP20 1534 1220 20.47
BP50 1461 1420 2.81
BP100 1495 1770 15.54
The operating point at 2500 rpm and 80 Nm was
used for calculating the NO
x
production thermal
mechanism due to its higher combustion diffusion
phase. The correlation was not used in the case of fuel
BP5 owing to its low combustion diffusion phase. In
Table 7 the results are summarized.
4 CONCLUSIONS
Palm oil biodiesel and its blends with diesel produce
variations in the NO
x
emissions, which increase or
decrease according to the engine operation
conditions. In general, at high loads the NO
x
emissions are increased. This behavior can be
explained for the high component of the diffusion
combustion phase. At low loads, the premixed
combustion phase is predominant, thus resulting in a
decrease of NO
x
emissions.
The correlations for determining the NO
x
formation should include parameters such as: Cetane
number, Iodine number in order to get a better
estimations taking into account the chemical and
physical features of the fuels used.
The combustion processes in diesel engines is
highly complex due to the high number of physical
and chemical interactions that occur during the
operation of the engine. Each phenomenon occurs in
tridimensional fluxes, turbulent and non-stationary,
interacting with a fuel conformed by complex chains
of hydrocarbons. In addition, a detailed chemistry
mechanism is unknown for the combustion of palm
oil biodiesel.
Lastly, it is necessary to implement optimization
techniques for parameter calibration between the
experimental and modeled values.
ACKNOWLEDGEMENTS
We would like to thank the GIMEL research group of
the Universidad de Antioquia in Medellin for the use
of the experimental facilities and advice. Also, we
would like to express our acknowledgments to
Universidad Cooperativa de Colombia and Istituto
Motori of the CNR for their financial support under
the project No. 1510 and the Cooperation Agreement
No. 0000720. COST (European Cooperation in
Science and Technology) Action FP1306 support is
also gratefully acknowledged.
REFERENCES
Agudelo, A. F., Agudelo, J. R. Benjumea, P. N., 2007.
Diagnostico de la combustion de biocombustibles en
motores, Universidad de Antioquia.
Agudelo, J., Bunjumea, P., Villegas, A. P., 2010.
Evaluation of nitrogen oxide emissions and smoke
opacity in a HSDI diesel engine fuelled with palm oil
biodiesel. Revista Facultad de Ingeniería Universidad
de Antioquia, 62-71.
Ban-Weiss, G. A., Chen, J. Y., Buchholz, B. A., Dibble, R.
W., 2007. A numerical investigation into the anomalous
slight NO
x
increase when burning biodiesel; A new
(old) theory. Fuel Processing Technology, 88, 659-667.
Dec, J. E., 1997. A conceptual model of di diesel
combustion based on laser-sheet imaging*. SAE
technical paper 970873, doi: 10.4271/970873.
Eckerle, W. A., Lyford-Pike, E. J., Stanton, D. W.,
Lapointe, L. A., Whitacre, S. D., Wall, J. C., 2009.
Effects of Methyl Ester Biodiesel Blends on NO
x
Emissions. SAE International Journal of Fuels and
Lubricants, 1, 102-118.
Fernando, S., Hall, C., Jha, S. 2006. NO
x
Reduction from
Biodiesel Fuels. Energy & Fuels, 20, 376-382.
Glaude, P.-A., Fournet, R., Bounaceur, R., Molière, M.,
2010. Adiabatic flame temperature from biofuels and
fossil fuels and derived effect on NO
x
emissions. Fuel
Processing Technology, 91, 229-235.
Guardiola, C., López, J. J., Martín, J. García-Sarmiento, D.,
2011. Semiempirical in-cylinder pressure based model
Experimental Analysis and Modeling of NO
x
Emissions in Compression Ignition Engines Fueled with Blends of Diesel and Palm Oil
Biodiesel
251

for NO
x
prediction oriented to control applications.
Applied Thermal Engineering, 31, 3275-3286.
Lapuerta, M., Armas, O., Hernández, J. J., 1999. Diagnosis
of DI Diesel combustion from in-cylinder pressure
signal by estimation of mean thermodynamic properties
of the gas. Applied Thermal Engineering, 19, 513-529.
Lapuerta, M., Armas, O., Rodríguez-Fernández, J., 2008.
Effect of biodiesel fuels on diesel engine emissions.
Progress in Energy and Combustion Science, 34, 198-
223.
Miller, J. A., Bowman, C. T., 1989. Mechanism and
modeling of nitrogen chemistry in combustion. Progress
in Energy and Combustion Science, 15, 287-338.
Park, W., Lee, J., Min, K., Yu, J., Park, S., Cho, S., 2013.
Prediction of real-time NO based on the in-cylinder
pressure in Diesel engines. Proceedings of the
Combustion Institute, 34, 3075-3082.
Sun, J., Caton, J. A., Jacobs, T. J., 2010. Oxides of nitrogen
emissions from biodiesel-fuelled diesel engines.
Progress in Energy and Combustion Science, 36, 677-
695.
Timoney, D. J., Desantes, J. M., Hernández, L., Lyons, C.
M., 2005. The development of a semi-empirical model
for rapid NO
x
concentration evaluation using measured
in-cylinder pressure in diesel engines. Proceedings of
the Institution of Mechanical Engineers, Part D:
Journal of Automobile Engineering, 219, 621-631.
Turns, S. R., 1996. An introduction to combustion,
McGraw-hill New York.
SFFEV 2016 - Special Session on Simulation of flex fuel engines and alternative biofuel vehicles
252
