
Electromyographic Signal Dynamic Behavior in Neuropathies
Spectral Parameters Evaluation and Classification
Maria Marta Santos
1,2
, Ana Lu
´
ısa Gomes
1,2
, Hugo Gamboa
1,2
, Mamede de Carvalho
3
, Susana Pinto
3
and Carla Quint
˜
ao
1,4
1
Departamento de Fsica, Faculdade de Ci
ˆ
encias e Tecnologia, Universidade Nova de Lisboa, Lisboa, Portugal
2
PLUX - Wireless Biosignals, Lisboa, Portugal
3
Faculdade de Medicina, Instituto de Medicina Molecular, Universidade de Lisboa, Lisboa, Portugal
4
Instituto de Biof
´
ısica e Engenharia Biom
´
edica, Faculdade de Ci
´
encias, Universidade de Lisboa, Lisboa, Portugal
Keywords:
Amyotrophic Lateral Sclerosis (ALS), Coherence, Phase Locking Factor (PLF), Fractal Dimension (FD),
Lempel-Ziv (LZ), Detrended Fluctuation Analysis (DFA), Multiscale Entropy (MSE) , Surface Electromyog-
raphy (sEMG), Ipsilateral, Classification.
Abstract:
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease characterized by motor neurons degen-
eration, which reduces muscular force, being very difficult to diagnose. Mathematical methods, such as
Coherence, Phase Locking Factor (PLF), Fractal Dimension (FD), Lempel-Ziv (LZ) techniques, Detrended
Fluctuation Analysis (DFA) and Multiscale Entropy (MSE) are used to analyze the surface electromiographic
signal’s chaotic behavior and evaluate different muscle groups’ synchronization. Surface electromiographic
signal acquisitions were performed in upper limb muscles, being the analysis executed for instants of contrac-
tion recorded from patients and control groups. Results from LZ, DFA and MSE analysis present capability
to distinguish between the patient and the control groups, whereas coherence, PLF and FD algorithms present
results very similar for both groups. LZ, DFA and MSE algorithms appear then to be a good measure of
corticospinal pathways integrity. A classification algorithm was applied to the results in combination with
extracted features from the surface electromiographic signal, with an accuracy percentage higher than 70%
for 118 combinations for at least one classifier. The classification results demonstrate capability to distin-
guish both groups. These results can demonstrate a major importance in the disease diagnose, once surface
electromyography (sEMG) may be used as an auxiliary diagnose method.
1 INTRODUCTION
ALS is a fatal and very progressive disease, character-
ized by both upper and lower motor neurons degen-
eration, involving brainstem and also multiple spinal
cord innervation regions. This disorder is responsi-
ble for abnormal motor activity. ALS patients typ-
ically present fatigue, quickly progressive weakness
and reduced exercise capacity with loss of voluntary
movement, spasticity, fasciculations, dysphagia (dif-
ficulty in swallowing), dyspnea (difficulties in breath-
ing) and dysarthria (difficulties in speaking). After the
first symptoms, death may occur within 3−5 years for
most of the patients. ALS is very difficult to diagnose,
since there isn’t available a reliable biomarker of dis-
ease activity and progression (Kiernan et al., 2011;
Mitchell and Borasio, 2007).
Upper motor neuron integrity can be evaluated
through the investigation of oscillatory activity prop-
agation. The motor cortex activity can be recorded,
and both alpha (8 − 12Hz) and beta (15 − 30Hz) fre-
quency bands can be analyzed via coherence and PLF
(Farmer et al., 2007).
This work explores the analysis of ipsilateral ac-
quisitions, which was presented with promissory pre-
liminary results in (Camara, 2013), using different ap-
proaches.
Motor unit recruitment patterns complexity can be
quantified using FD. However, the strength of a mus-
cle’s contraction is better estimated based on Max-
imum Fractal Length (MFL), even for very small
muscle contraction strength, rather than FD (Poos-
apadi Arjunan and Kumar, 2012).
The LZ measure is a well suited feature regard-
227
Marta Santos M., Luisa Gomes A., Gamboa H., Carvalho M., Pinto S. and Quintão C..
Electromyographic Signal Dynamic Behavior in Neuropathies - Spectral Parameters Evaluation and Classification.
DOI: 10.5220/0005215602270234
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2015), pages 227-234
ISBN: 978-989-758-069-7
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

ing sEMG analysis, particularly during dynamic con-
tractions (highly non-stationary signal), since this
feature doesn’t make any assumption of stationarity
(Talebinejad et al., 2011).
The DFA method is proved to be efficient to de-
scribe upper-limbs movements. DFA outperforms
other methods such as correlation dimension and
Higuchi methods, being a stable technique to quan-
tify fractality and to establish self-similarity, being a
robust method in the presence of nonstanionarity time
series and trends (Phinyomark et al., 2011).
Entropy is a feature which can detect and quantify
differences in the EMG signal amplitude distributions
due to neuromuscular conditions (pathology) (Kapla-
nis et al., 2010). This feature has been successfully
applied to physiological signals, in order to quantify
their degree of complexity. Multiscale entropy (MSE)
has been proved to be more effective than single-scale
entropy in this quantification, since it considers mul-
tiple spatiotemporal scales (Zhang et al., 2013).
k-Nearest Neighbor is a relatively simple and fast
algorithm, important characteristics in the classifica-
tion process (Kim et al., 2011). Decision tree meth-
ods have also been used for dealing with classification
problems in various domains, such as pattern recog-
nition, data mining, web mining and signal process-
ing, among others. However, standard decision tree
algorithms can only handle discrete attributes (Wang
et al., 2006). The decision tree algorithm has usu-
ally good performance for large data sets in a short
time (Zhang et al., 2011). Random Forest algorithm
has been tested with real and simulated data sets. The
results have been proven to be very accurate. This al-
gorithm is fast, versatile and can be applied to very
large data sets. It has also been shown its robustness
against noise in the outcome compared with several
other methods (Roy and Larocque, 2012). Discrim-
ination between subject or patient group concerning
age, gender and injuries in athletes has been proven
to be effective using a classification approach with
generic features and AdaBoost (Eskofier et al., 2012).
Na
¨
ıve Bayes method has shown to be competitive
among much sophisticated induction algorithms con-
cerning experiments on real world data, despite the
assumption of conditional independence (Wang et al.,
2006).
2 METHODS
2.1 Coherence
Coherence function estimates values from 0 to 1, as-
suming the value 0 if there is no association between
two signals at a certain frequency, and the value 1 if
there is a perfectly linear association between them
(Farmer et al., 2007)
2.2 Phase Locking Factor (PLF)
PLF is a measure of synchronization between two
signals, in which the frequencies of interest are iso-
lated by the application of a narrow band-passed filter.
Then, the relationship between the phases of the two
signals, φ
j
(t) and φ
k
(t) are analyzed (Almeida et al.,
2011):
ρ
jk
≡ |
1
T
T
∑
t=1
e
i[φ
j
(t)−φ
k
(t)]
| = |he
i[φ
j
(t)−φ
k
(t)]
i| (1)
where ρ
jk
is the PLF and T is the number of discrete
samples.
PLF ranges from 0 to 1. While ρ
jk
= 0 corre-
sponds to asynchronous signals (their phases are not
correlated), ρ
jk
= 1 is attained if the two signals are
in perfect synchronization (Almeida et al., 2011).
2.3 Fractal Dimension (FD)
FD is one of the most used measurements for the eval-
uation of the dynamics of complex systems. A non-
integer FD usually indicates a chaotic behavior, and
the smallest integer bigger than FD is considered the
minimum number of independent variables capable to
describe this behavior (West, 1994).
For physiological signals, FD can be estimated us-
ing Higuchi algorithm, since it is suitable for non-
periodic and irregular time series.
This algorithm results on a plot of several curve
lengths, L(k), for a pre-determined k
max
, being k ∈
[1,k
max
] (Phinyomark et al., 2011).
2.4 Lempel-Ziv (LZ)
LZ is another tool used to analyze the deterministic
complexity of a highly non-linear chaotic setting. To
compute LZ, it is necessary to convert the sEMG sig-
nal into a symbolic sequence, conventionally a binary
sequence, by comparison to a threshold. Next, the
number of distinct patterns within a signal is obtained,
which is directly related to the complexity of the sys-
tem (Talebinejad et al., 2011).
2.5 Detrended Fluctuation Analysis
(DFA)
The DFA method can be applied to the study of elec-
trophysiological signals, being a modified root mean
BIOSIGNALS2015-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
228

square (RMS) analysis of a random walk (Phiny-
omark et al., 2011).
The outputs of the DFA algorithm are scaling ex-
ponents extracted from a log-log graph (for details
(Phinyomark et al., 2011)). These exponents assume
values between 0 and 2, according to the time series
behavior:
• If 0 < α <
1
2
the time series is anti-correlated
• If α
∼
=
1
2
the time series is uncorrelated, or indi-
cates White noise (the value at one instant cannot
be correlated with any previous value)
• If
1
2
< α < 1 the time series is correlated
• If α
∼
=
1 indicates Pink noise (
1
f
noise)
• If 1 < α <
3
2
indicates nonstationary or random
walk
• If α
∼
=
3
2
indicates Brownian noise (i. e. the inte-
gration of the White noise)
2.6 Multiscale Entropy (MSE)
Entropy has been often used to quantify complexity,
since traditional entropy definitions (e. g. Shannon-
entropy) are used to measure disorder and uncertainty,
as well as to characterize a systems’ gain of informa-
tion. Approximate Entropy and its modification Sam-
ple Entropy are entropy-based complexity measures
with a single scale which are widely used in short
and noisy time series. Thus, in MSE one of this ap-
proaches is applied to different time scales, resulting
on a plot of the entropy value as a function of the fac-
tor scale, τ (Zhang et al., 2013).
2.7 Classification
Classification methods are used to identify the be-
longing of a novel observation in a set of categories
(sub-populations). These categories are obtained with
based on a training group of observations, therefore,
it is necessary to have previous knowledge of the cat-
egory membership of each observation of the train-
ing group. These observations are then analyzed
according to the extrated features. In this project,
the used features are: kurtosis, maximum frequency,
mean, median frequency, power band, spectral kur-
tosis, spectral skewness, spectral spread and correla-
tion. There will be also analyzed the results of the pre-
viously referred implemented algorithms (coherence,
PLF, FD, LZ, DFA and MSE). Classification can be
implemented throughout a various number of algo-
rithms, the classifiers (k - Nearest Neighbor, Decision
Tree, Random Forest, AdaBoost and Na
¨
ıve Bayes).
Leave-one-out cross validation iterator is used to split
data in train/test sets. Hence, all samples except one
are used as a train set, being this left out sample tested
after (Pedregosa et al., 2011).
3 ACQUISITIONS
3.1 Subjects
Measurements were performed in two different
groups of subjects: group of patients, with 21 mem-
bers presenting ALS, and group of control, with 26
members which do not evidence ALS disease. There-
fore, the patients group contains 21 subjects, varying
this number according to the analyzed channel (left or
right hand or forearm), since some patients presented
inability of self-controlled movement for one arm. All
participants from the patients group have been diag-
nosed within less than three years, except two mem-
bers which have been diagnosed previously. All par-
ticipants from both groups have ages between 23 and
77 years (mean of 59 years for the patient group and
45 years for the control group).
3.2 Acquisition Protocol
The acquisition protocol is identical to the one in (Ca-
mara, 2013). The performed task was repeated for 6
minutes or less according to maximum time borne by
the patients. Subjects sat down and placed both hands
and forearms on a desk in a parallel position, 10 cm
away from each other with hand palms facing one an-
other in 90 degrees flexion with the elbow. While
listening to a programmed sound, which guided the
movement, subjects were asked to coordinately ele-
vate both index fingers vertically with maximum ar-
ticular amplitude in an opposite direction from the
other fingers position, hold that position for 3 sec-
onds while maintaining a certain force and return to
the original position, remaining in that position for
3 seconds while trying to relax the arms muscles as
much as possible.
3.3 Recordings
Contralateral and ipsilateral acquisitions were
recorded simultaneously, and 4 signals were acquired
from each subject using EMG sensors attached to
a bioPlux device. The sensors were placed on the
first dorsal interosseus muscle for both left and right
hand, and on the extensor digitorum communis
muscle for both left and right forearm. Ground was
placed on ulna bone inferior extremity, where no
ElectromyographicSignalDynamicBehaviorinNeuropathies-SpectralParametersEvaluationandClassification
229

muscle activity is present. Figure 1 shows the surface
electrodes placements.
The bioPlux device has eight analog channels with
12-bit resolution and also an external channel to be
used as a reference ground. The EMG sensors have
second order band pass filter with cutoff frequencies
of 25 and 450. EMG signals were recorded using a
sampling frequency of 1000 Hz, being the recorded
data transmitted via Bluetooth to a computer.
Figure 1: Simultaneous contralateral and ipsilateral exper-
imental setup: Bioplux research device, placement of four
EMG sensors and ground.
4 SIGNAL PROCESSING
All acquired signals were processed using python lan-
guage. First, the signals’ Direct Current (DC) compo-
nent was removed and a third order butter band pass
filter of 10 − 500 Hz was applied. From each pair
of ipsilateral signals, intervals of common contraction
were isolated from intervals of relaxation using the al-
gorithm referred in (Camara, 2013).
All the previously referenced algorithms were ap-
plied to moments of contraction. While coherence
and PLF were calculated twice for each subject, be-
ing calculated for a pair of signals, FD, LZ, DFA
and MSE were calculated four times, being applied
to each one of the four acquired signals individually.
Coherence and PLF were calculated for two com-
mon sections of data from an interval of one contrac-
tion. This was performed for all contractions, with
posterior averaging of all epochs. FD, LZ, DFA and
MSE calculus was applied to a concatenation of all
contractions.
4.1 Coherence Processing
All EMG signals were full-wave rectified and then,
for each contraction, coherence was calculated using
python libraries (matplotlib.mlab.cohere tool). Coher-
ence is then averaged for the moments of contrac-
tion. The used sampling frequency is 1000 Hz, the
Nonequispaced fast Fourier transform (NFFT) is 512
and the value that dictates the dependency between
FFT windows is NFFT/2. Coherence was averaged
for each frequency among all the subjects within the
same group.
4.2 PLF Processing
PLF algorithm was developed in (Camara, 2013).
All signals were full-wave rectified and each signal
was band pass filtered in order to remove all the
other undesirable frequencies. The used filter was
[ f − 2, f + 2], being f the analyzed frequency. There-
fore, PLF calculus was performed as many times as
the number of frequencies to analyze. PLF was ob-
tained with resolution of 1Hz for all the frequencies
within the beta band (15−30Hz). PLF was computed
according to equation 1. Hence, a different PLF value
is obtained for each contraction of the analyzed sig-
nals and, in order to attain a final value for each sub-
ject, PLF was averaged among all contractions within
the same acquisition. Finally, for each analyzed fre-
quency, PLF was averaged among all members within
each group, for the patient and the control groups.
4.3 FD Processing
In the Higuchi fractal dimension algorithm k
max
was
defined as 128, as suggested in literature (Phinyomark
et al., 2011). The FD coefficient was estimated and
averaged among all subjects within each group.
4.4 LZ Processing
The LZ coefficient was calculated for a binary se-
quence obtained from the rectified filtered used signal
with threshold defined as 0.4.
Since the LZ coefficient is greatly related to the
number of different patterns within each signal, and
the number of patterns is related to the length of the
signal, all the signals were cut in accordance with
the minimum reasonable signal length within both
groups. An average of the LZ coefficient was cal-
culated for all members within the patients and the
control’s groups.
4.5 DFA Processing
The DFA was applied among all subjects, obtaining
two distinct scaling exponents, α
1
and α
2
. These scal-
ing exponents were averaged for all members within
each group.
BIOSIGNALS2015-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
230
4.6 MSE Processing
For MSE, the Sample Entropy was computed for 20
scales. These values were averaged among all sub-
jects within each group, and plotted in a graph for
both populations.
4.7 Classification
The feature extraction and the classification algo-
rithms were developed in (Gomes, 2014).
The algorithms were adapted for the use of two
channels, and the features extracted were selected ac-
cording to this work objectives.
Features were extracted from a pair of signals
(right hand and forearm). The sampling frequency
was placed at 1000 Hz, being used a central window
with 2000 points. The results obtained from the pre-
viously described algorithms were joined to these fea-
tures.
Posteriorly several combinations of the extracted
features, the developed algorithm results and of both
of them were organized and classified.
5 RESULTS AND DISCUSSION
In this section, we show results obtained from right
arm signals, being the most relevant conclusions also
achieved for left arm analysis.
5.1 Coherence Results
Coherence mean values for the group of patients and
the group of control are presented in Figure 2.
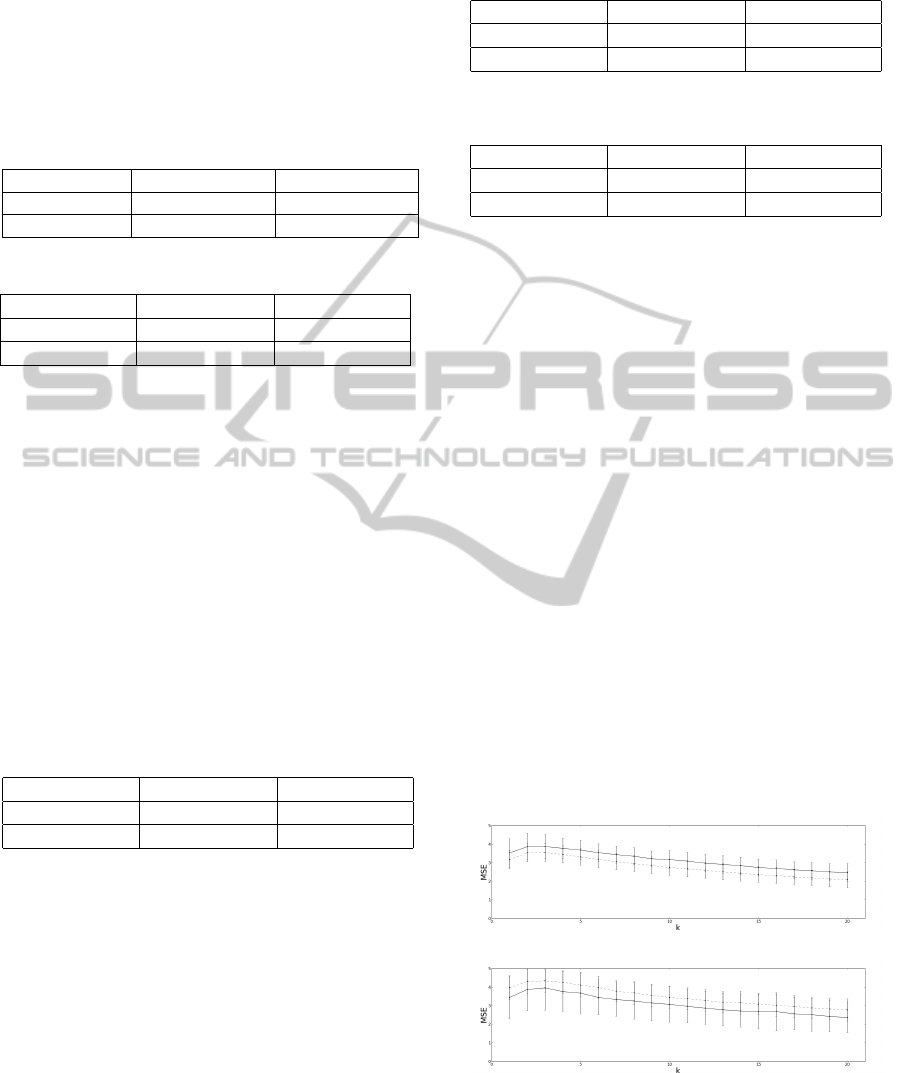
Figure 2: Mean coherence dependency on frequency for pa-
tients by the straight line and for controls by the dashed line
with NFFT placed as 512. The first grey box delimitates
the frequencies corresponding to the alpha band (8 − 12
Hz). The second grey box delimitates the frequencies cor-
responding to the beta band (15 − 30 Hz). These results are
from the right arm.
By the observation of the graphics presented in
Figure 2, coherence mean values are very similar
for both the patients and the control groups. Con-
trol group mean value for coherence is slightly higher
within the alpha band. Coherence pooled value for
patients is 0,23 ± 0,21 for the right arm. Coherence
pooled value for the control group is 0, 22 ± 0,21 for
the right arm. These values are different from those
found in literature (Fisher et al., 2012), since slightly
higher values of coherence were expected for the con-
trol group within the beta band than for the patients
group. These differences may be explained by the
sampling frequency used, differences in the acquisi-
tion protocol, differences in the used algorithm or pa-
rameters and the tested subjects themselves (age, gen-
der, lifestyle, etc.). The obtained results are also dif-
ferent from the expected for ipsilateral acquisitions in
(Camara, 2013), since only preliminary results were
obtained previously.
5.2 PLF Results
PLF mean values depending on frequency for both
groups are presented in Figure 3.
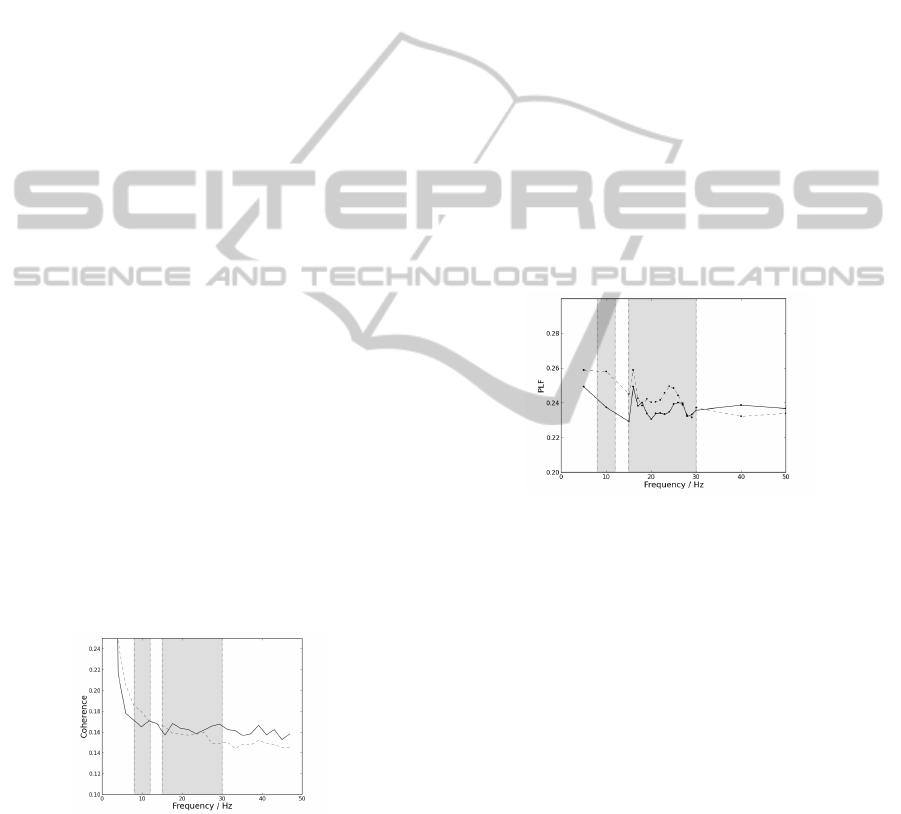
Figure 3: Mean PLF values dependency on frequency for
patients by the straight line and for controls by the dashed
line; The first grey box delimitates the frequencies corre-
sponding to the alpha band (8 − 12 Hz). The second grey
box delimitates the frequencies corresponding to the beta
band (15 − 30 Hz). These results are from the right arm.
Observing the graphics presented in Figure 3, PLF
values appear to be very similar for both groups. Al-
pha band appears to demonstrate a higher difference
between both groups PLF values. However, since
this work proceeds the investigation of PLF within
the beta band (Camara, 2013), PLF was only com-
puted for 10 Hz within the alpha band. PLF pooled
value for the patients group is 0, 237 ± 0,006 for the
right arm. PLF pooled value for the control group is
0,242 ±0,009 for the right arm. These results are dif-
ferent from the expected for ipsilateral acquisitions in
(Camara, 2013), since only preliminary results were
obtained previously. Differences in results may also
be explained by the length of the used signal and the
sampling frequency used, and characteristics such as
age, gender and lifestyle may influence this analysis.
ElectromyographicSignalDynamicBehaviorinNeuropathies-SpectralParametersEvaluationandClassification
231
5.3 FD Results
FD coefficient mean and standard deviation values are
presented in Table 1, for the group of patients and the
group of control. Table 2 represents the MFL (the
point of lowest scale) values for each group.
Table 1: Mean and standard deviation values of FD coeffi-
cient for patients and control group for the right arm.
Patients group Control group
Right hand −1.985 ± 0.008 −1.983 ± 0.008
Right forearm −1.983 ± 0.013 −1.984 ± 0.006
Table 2: MFL values for patients and control group.
Patients group Control group
Right hand 3.72 ± 0.39 3.99 ± 0.20
Right forearm 3.72 ± 0.47 3.80 ± 0.22
From the observation of Table 1 FD coefficient is
almost identical for both groups. Therefore, FD co-
efficient is not a good measure of distinction between
patients and control group. However, the obtained FD
values are very similar to the results obtained from
different upper limb activities in (Phinyomark et al.,
2011). Observing Table 2, MFL is always slightly
higher for the control group.
5.4 LZ Results
Table 3 shows the LZ coefficient mean value and stan-
dard deviation for a binary sequence.
Table 3: LZ coefficient for a binary sequence obtained from
the rectified filtered used signal with threshold defined as
0.4.
Patients group Control group
Right hand 0.17 ± 0.13 0.27 ± 0.16
Right forearm 0.27 ± 0.29 0.15 ± 0.13
From the observation of Table 3, LZ coefficient
presents higher values for the control group for the
right hand, and higher values for the patients group for
the right forearm. Tables 3 presents a good distinction
between patient and control groups. Therefore, for a
binary sequence obtained from the filtered used signal
and for a binary sequence obtained from the rectified
filtered used signal, LZ algorithm appears to distin-
guish subjects between both groups regarding pooled
LZ coefficient values, however, with some variance
among subjects within each group.
Table 4: DFA α
1
coefficient mean and standard deviation
values for both groups for the right arm.
Patients group Control group
Right hand 1.23 ± 0.17 1.35 ± 0.14
Right forearm 1.39 ± 0.09 1.44 ± 0.11
Table 5: DFA α
2
coefficient mean and standard deviation
values for both groups for the right arm.
Patients group Control group
Right hand 0.50 ± 0.12 0.52 ± 0.09
Right forearm 0.62 ± 0.16 0.59 ± 0.10
5.5 DFA Results
Tables 4 and 5 present the DFA coefficients, α
1
and
α
2
mean values for both groups, respectively.
As observed in Tables 4 and 5, DFA coefficients
generally present higher values for the control group.
Therefore, DFA algorithm appears to distinguish both
groups.
5.6 MSE Results
Figure 4 represents the Sample Entropy mean value
for each scale for both patient and control groups.
Observing Figure 4, patients group exhibit higher
entropies for the right hand, whereas control group
exhibit higher entropies for the right forearm. There-
fore, despite statistically the differences between pa-
tients and control’s groups are very small, the MSE
tendency appears to be distinct between both groups.
MSE pooled values for the patients and control
groups are presented in Table 6.
Observing the results presented in Figure 4 and
Table 6, MSE algorithm appears to be capable of dis-
tinguish both groups.
(a)
(b)
Figure 4: Sample Entropy mean value for each scale. The
straight line represents the patient group, and the dashed
line represents the control group. The errorbars represent
the standard deviation. (a) Results for the right hand. (b)
Results for the right forearm.
BIOSIGNALS2015-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
232

Table 6: MSE mean and standard deviation values, for both
patients and control groups.
Patients group Control group
Right hand 3.15 ± 0.46 2.77 ± 0.48
Right forearm 3.08 ± 0.49 3.50 ± 0.52
5.7 Classification Results
Table 7 presents the 14 combinations and the 3 classi-
fiers with better results for the right arm for a Leave-
one-out cross validation.
Table 7: Classification results for the right arm for Deci-
sion Tree, Random Forest and AdaBoost Classifiers. 1) sum
power band + MSE + DFA (α
1
+ α
2
); 2) mean + MSE +
DFA (α
1
+ α
2
); 3) mean + MSE + DFA (α
1
+ α
2
) + LZ; 4)
spectral skewness + MSE + DFA α
1
+ α
2
) + LZ; 5) spectral
spread + MSE + DFA (α
1
+ α
2
) + LZ; 6) spectral kurtosis
+ MSE + DFA (α
1
+ α
2
) + LZ; 7) sum power band + MSE
+ coherence + PLF + DFA (α
1
+ α
2
); 8) kurtosis + MSE
+ DFA (α
1
+ α
2
); 9) kurtosis + MSE + DFA (α
1
+ α
2
) +
LZ; 10) kurtosis + MSE + coherence + PLF + DFA (α
1
+
α
2
); 11) maximum frequency + MSE + coherence + PLF
+ DFA (α
1
+ α
2
); 12) mean +MSE + DFA (α
1
+ α
2
); 13)
spectral skewness + MSE + coherence + DFA (α
1
+ α
2
);
14) spectral spread +MSE + coherence + PLF + DFA (α
1
+
α
2
).
Combinations Decision Tree Random Forest AdaBoost
Right Hand
1) 0,806 0,694 0,722
2) 0,806 0,778 0,722
3) 0,833 0,611 0,750
4) 0,806 0,667 0,667
5) 0,806 0,639 0,722
Right Forearm
6) 0,667 0,750 0,806
Right Arm
7) 0,778 0,750 0,806
8) 0,556 0,750 0,806
9) 0,500 0,806 0,778
10) 0,694 0,833 0,694
11) 0,667 0,611 0,806
12) 0,556 0,806 0,639
13) 0,667 0,861 0,750
14) 0,694 0,833 0,694
Classification results demonstrate the algorithms
distinction capability, since the presented results show
the percentage of cases that the algorithm classified
correctly (accuracy percentage).
Classification results were obtained for the right
arm for a leave-one-out cross validation tested with
k - Nearest Neighbor, Decision Tree, Random For-
est, AdaBoost and Na
¨
ıve Bayes classifiers. In spite
of 201 combinations have been arranged, 118 combi-
nations present values higher than 70.0% for at least
one classifier, 41 combinations present results higher
than 77,8% for at least one classifier, and 14 combina-
tions present results higher than 80.6% for one classi-
fier (Decision Tree, Random Forest or AdaBoost).
The best results include MSE, DFA, LZ, coher-
ence and PLF algorithm results, and also the extracted
features mean, maximum frequency, spectral kurto-
sis, spectral skewness, spectral spread, sum power
band and kurtosis, in several different combinations.
The top 14 best combinations include the results of
both DFA and MSE algorithms. The best obtained
combination is spectral skewness + MSE + coher-
ence + DFA (α
1
and α
2
), with an accuracy percent-
age of 86,1% for a Random Forest Classifier. There-
fore, it is proved that in spite of some algorithms
may present slender differences between both control
and patients groups, the combination of these algo-
rithms with other measures can improve the distinc-
tion capability between members of patients and con-
trol groups.
6 CONCLUSIONS
In this work, methodology was developed to evalu-
ate the complexity of sEMG signal acquired from dif-
ferent muscle groups of healthy subjects and patients
with ALS. FD, LZ, DFA and MSE algorithms were
implemented and all of these algorithms as well as
coherence and PLF algorithms were applied to the ac-
quired filtered signals.
Contrary to the results presented in literature, co-
herence analysis does not present significant differ-
ences between the group of patients and the group
of control. PLF analysis also does not present any
significant differences between both groups. Results
from both algorithms appear to be slightly higher for
the control group for the alpha band frequencies. For
further work is suggested to compute PLF for all the
frequencies within the alpha band with resolution of
1 Hz.
FD analysis results in FD coefficients very sim-
ilar for all the signals for both patients and control
groups. Therefore, this algorithm does not seem good
to obtain a distinction between both groups. How-
ever, MFL presents slightly higher values regarding
the control group, being this a better measure of dis-
tinction between both groups than the FD coefficient.
LZ analysis presents better results, being LZ coef-
ficient higher for the control group for the right hand,
and higher for the patients group for the right fore-
arm. These results are for a binary sequence obtained
from the rectified filtered used signal. Therefore, LZ
coefficient appears to be a good reflection of neural
degeneration. Since the used threshold to obtain the
binary sequence was defined as 0.4, it is not adapted
ElectromyographicSignalDynamicBehaviorinNeuropathies-SpectralParametersEvaluationandClassification
233

to each individual signal. Therefore, it is suggested
for further work a threshold obtained as a percentage
of the standard deviation of each signal.
DFA analysis presents higher values for both α
1
and α
2
DFA coefficients for the control group. Then,
this algorithm seems to be a good measure to reflect
neural degeneration.
MSE analysis presents higher values for the right
hand for the patients group, and higher values for the
right forearm for the control group. This algorithm
appears to be a good indicator of neural degeneration.
LZ, DFA and MSE analysis have then potential as
a quantitative test for upper and lower neural integrity
concerning ALS disease.
Classification results demonstrate to provide a
good distinction of both groups, being the combina-
tion of various algorithms with features proved to be
advantageous to improve both groups distinction ca-
pability.
REFERENCES
Almeida, M., Vig
´
ario, R., and Bioucas-Dias, J. (2011).
Phase locked matrix factorization. In Proc. of the EU-
SIPCO Conference, pages 1728–1732.
Camara, M. (2013). Coherence and phase locking dis-
ruption in electromyograms of patients with amy-
otrophic lateral sclerosis. Master’s thesis, Faculdade
de Ci
ˆ
encias e Tecnologia da Universidade Nova de
Lisboa.
Eskofier, B. M., Kraus, M., Worobets, J. T., Stefanyshyn,
D. J., and Nigg, B. M. (2012). Pattern classifica-
tion of kinematic and kinetic running data to distin-
guish gender, shod/barefoot and injury groups with
feature ranking. Computer methods in biomechanics
and biomedical engineering, 15(5):467–474.
Farmer, S. F., Gibbs, J., Halliday, D. M., Harrison, L. M.,
James, L. M., Mayston, M. J., and Stephens, J. A.
(2007). Changes in emg coherence between long and
short thumb abductor muscles during human develop-
ment. The Journal of physiology, 579(2):389–402.
Fisher, K. M., Zaaimi, B., Williams, T. L., Baker, S. N.,
and Baker, M. R. (2012). Beta-band intermuscular
coherence: a novel biomarker of upper motor neu-
ron dysfunction in motor neuron disease. Brain,
135(9):2849–2864.
Gomes, A. L. (2014). Human activity recognition with ac-
celerometry: Novel time and frequency features. Mas-
ter’s thesis, Faculdade de Ci
ˆ
encias e Tecnologia da
Universidade Nova de Lisboa.
Kaplanis, P. A., Pattichis, C. S., Zazula, D., et al.
(2010). Multiscale entropy-based approach to au-
tomated surface emg classification of neuromuscular
disorders. Medical & biological engineering & com-
puting, 48(8):773–781.
Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R.,
Eisen, A., Hardiman, O., Burrell, J. R., and Zoing,
M. C. (2011). Amyotrophic lateral sclerosis. The
Lancet, 377(9769):942–955.
Kim, K. S., Choi, H. H., Moon, C. S., and Mun, C. W.
(2011). Comparison of¡ i¿ k¡/i¿-nearest neighbor,
quadratic discriminant and linear discriminant analy-
sis in classification of electromyogram signals based
on the wrist-motion directions. Current Applied
Physics, 11(3):740–745.
Mitchell, J. D. and Borasio, G. D. (2007). Amyotrophic
lateral sclerosis. The lancet, 369(9578):2031–2041.
Pedregosa, F., Varoquaux, G., Gramfort, A., Michel, V.,
Thirion, B., Grisel, O., Blondel, M., Prettenhofer,
P., Weiss, R., Dubourg, V., Vanderplas, J., Passos,
A., Cournapeau, D., Brucher, M., Perrot, M., and
Duchesnay, E. (2011). Scikit-learn: Machine learning
in Python. Journal of Machine Learning Research,
12:2825–2830.
Phinyomark, A., Phukpattaranont, P., Limsakul, C., and
Phothisonothai, M. (2011). Electromyography (emg)
signal classification based on detrended fluctuation
analysis. Fluctuation and Noise Letters, 10(03):281–
301.
Poosapadi Arjunan, S. and Kumar, D. K. (2012). Compu-
tation of fractal features based on the fractal analysis
of surface electromyogram to estimate force of con-
traction of different muscles. Computer Methods in
Biomechanics and Biomedical Engineering, (ahead-
of-print):1–7.
Roy, M.-H. and Larocque, D. (2012). Robustness of ran-
dom forests for regression. Journal of Nonparametric
Statistics, 24(4):993–1006.
Talebinejad, M., Chan, A. D., and Miri, A. (2011). A
lempel–ziv complexity measure for muscle fatigue es-
timation. Journal of Electromyography and Kinesiol-
ogy, 21(2):236–241.
Wang, L.-M., Li, X.-L., Cao, C.-H., and Yuan, S.-M.
(2006). Combining decision tree and naive bayes for
classification. Knowledge-Based Systems, 19(7):511–
515.
West, B. J. (1994). Fractal physiology, volume 2. Oxford
University Press.
Zhang, X., Chen, X., Barkhaus, P. E., and Zhou, P. (2013).
Multiscale entropy analysis of different spontaneous
motor unit discharge patterns. Journal of Biomedical
and Health Informatics, 17(2).
Zhang, X., Chen, X., Li, Y., Lantz, V., Wang, K., and Yang,
J. (2011). A framework for hand gesture recognition
based on accelerometer and emg sensors. Systems,
Man and Cybernetics, Part A: Systems and Humans,
IEEE Transactions on, 41(6):1064–1076.
BIOSIGNALS2015-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
234
