
The Ideomotor Principle Simulated
An Artificial Neural Network Model for Intentional Movement
and Motor Learning
Neri Accornero and Marco Capozza
Neuroscience Department, Sapienza University, Viale dell’Università 30, 00185, Rome, Italy
Keywords: Ideomotor Principle, Intentional Movement, Motor Learning, Artificial Neural Network, Simulation.
Abstract: Although the ideomotor principle (IMP), the notion positing that the nervous system initiates voluntary
actions by anticipating their sensory effects, has long been around it still struggles to gain widespread
acknowledgement. Supporting this theory, we present an artificial neural network model driving a simulated
arm, designed as simply as possible to focus on the essential IMP features, that demonstrates by simulation
how the IMP could work in biological intentional movement and motor learning. The simulation model
shows that IMP motor learning is fast and effective and shares features with human motor learning. An IMP
extension offers new insights into the so-called mirror neuron and canonical neuron systems.
1 INTRODUCTION
The ideomotor principle (IMP) claims that the
nervous system initiates voluntary actions by
anticipating their typical sensory consequences
(Kiesel and Hoffmann 2004, Stock and Stock 2004).
Over the past twenty years increasing evidence
favoring this theory emerged from both behavioral
studies (reviews in Wulf and Prinz, 2001;
Wohlschläger et al., 2003; Shin et al., 2010;
Hommel, 2013) and fMRI studies (Eran Dayan et
al., 2007; Melcher et al., 2008, 2013; Pfister et al.,
2014). Unfortunately these findings centered on
individual IMP features and they provided no overall
view of a working IMP. Given this background,
simulations with artificial neural networks (ANN)
that demonstrate how the IMP works may lead to its
wider acknowledgement. Although few reported
simulations of this type explicitly mention IMP
(Karniel and Inbar, 1997; Sauser and Billard, 2006;
Butz et al., 2007; Galtier, 2014), many involve
IMP’s underlying rationale, namely sensorimotor
mapping. Existing simulations nevertheless provide
scarce help in understanding the IMP because they
use non-IMP procedures, such as supervised
learning or complicated modularity and flowcharts,
or they add complex details that make the essential
IMP features even harder to understand.
In this paper we take an opposite approach: to
highlight how the IMP works and make it easy to
understand, we present an unsupervised ANN
system that is as simple and basic as possible and
learns to move a three-joint arm in a workspace
using the IMP and sensorimotor mapping. We
examine its main features and compare them with
those of human motor learning. We then suggest
how an IMP extension also offers new ways to look
at the so-called mirror neuron system.
2 METHODS
2.1 Model Design
Our simulation consisted of an ANN controlling a
three-joint simulated limb moving on a two-
dimensional plane (Fig. 1). The network received on
its input units sensory information on the limb
position, and sent limb commands from its output
units. Each limb movement was defined by three
vectors: the initial sensory state (before the
movement); the final sensory state (after the
movement); and the neuromuscular activations
needed to pass from the initial to the final state. The
first two vectors were given to the ANN input units,
and the ANN had to compute the third vector on its
output units. Because IMP states that intentional
limb movements depend on anticipation of their
226
Accornero N. and Capozza M..
The Ideomotor Principle Simulated - An Artificial Neural Network Model for Intentional Movement and Motor Learning.
DOI: 10.5220/0005081002260233
In Proceedings of the International Conference on Neural Computation Theory and Applications (NCTA-2014), pages 226-233
ISBN: 978-989-758-054-3
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
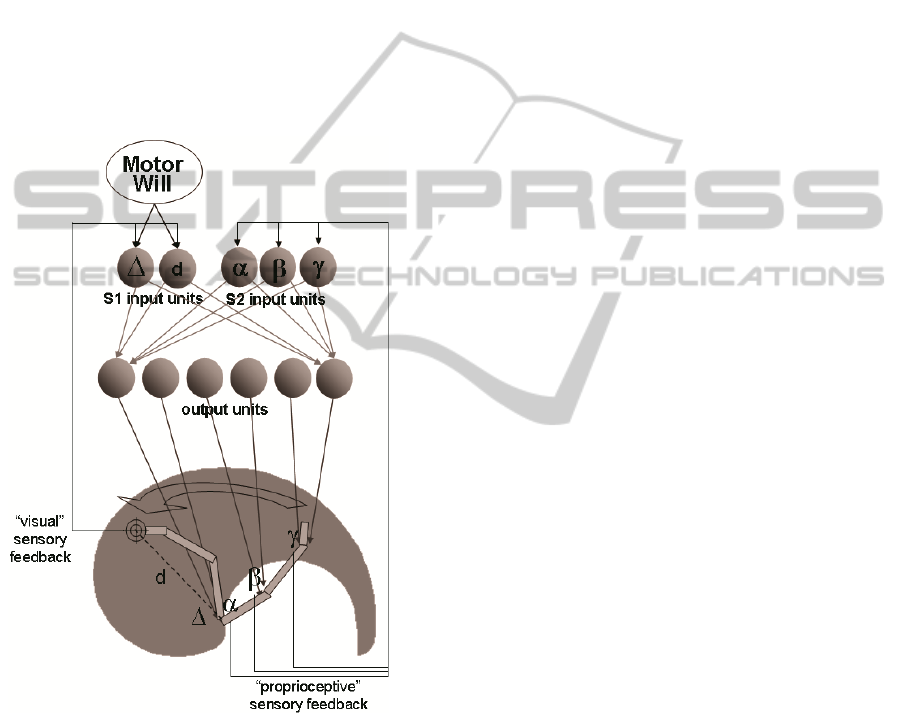
sensory effects, the ANN input units receiving
sensory information on the final limb state (S1 units)
also received motor commands from a component
outside the network that established where the
moving hand should be positioned and therefore
acted as Motor Will. Commands from it to the ANN
consisted of sensory representations of the desired
final hand position, coded as visuospatial
coordinates in agreement with the observation that
motion planning in human takes place in the visually
perceived space (Flanagan and Rao 1995, Shadmehr
2005). Unlike S1 units, the ANN input units
receiving sensory information on the initial limb
state (S2 units) did not receive motor commands,
they only received “proprioceptive” sensory
information from the limb joint angles.
Figure 1: General architecture for the Ideomotor Principle
(IMP) simulation model. The artificial neural network
(ANN) controls a 3-joint limb moving in a two-
dimensional plane. The ANN receives sensory feedback
information on the limb and motor commands from Motor
Will. Δ, d: polar coordinates for the hand (Δ = angle with
respect to the posterior-anterior axis, d = distance from the
shoulder point). α, β, γ: shoulder, elbow, wrist joint angle.
Not all input-to-output connections depicted; actually each
input unit sends connection to all output units.
Given that velocity information was not
indispensable to the key IMP mechanism as long as
the limb was assumed to start from still and end still,
we decided to give the ANN only sensory
information about limb position (joint angles and
spatial hand position), not velocity.
2.1.1 Limb
The limb was designed to represent a simplified
model of the human right arm comprising three
segments, “arm”, “forearm” and “hand” articulated
with three joints “shoulder”, “elbow” and “wrist”,
with the shoulder situated in a fixed point in space,
and the hand able to move freely in the reachable
space. The arm measured 70 pixels in length, the
forearm 70 pixels and the hand 20 pixels (because
the model involved a simulation displayed on a
computer screen, for simplicity lengths are given in
pixels). The three joints opened and closed within
angular limits in a similar way to a human arm: the
shoulder from 23 to 190 degrees, the elbow from 20
to 180, the wrist from -90 to 72. The overall area
reachable with the hand (grey area in Fig. 1)
therefore assumed a drop-like shape measuring 298
x 200 pixels.
The three joints were each controlled by an
agonist-and-antagonist muscle couple. Each muscle
was controlled by a neural network output unit.
Muscle actions were simulated in a simplified
manner, without recourse to spring models or
tension-length diagrams. Analog outputs from 0 to 1
from the two units acting on muscle flexion and
extension for every joint were assumed to determine
variations in joint opening or closing according to
the following equations:
a
j,
t+1
= a
j,t
+ (e
j,t
– f
j,t
+ p
j,t
) / m
j
(1)
p
j,t
= [ (amax
j
+ amin
j
) / 2 - a
j,t
] · k
j
(2)
where:
a
j,t
= degree of joint j opening at time t, in radians;
e
j,t
= output (0 to 1) from the unit controlling the
extensor muscle for joint j at time t;
f
j,t
= output (0 to 1) from the unit controlling the
flexor muscle for joint j at time t;
p
j,t
= passive elastic muscle and ligament force
acting on the joint j, maximum when the joint is
fully opened or closed;
m
j
= mass in the segment distal to the joint j,
normalized for the upper arm (1 for the upper arm,
0.6 for the forearm, 0.2 for the hand);
amax
j
= degree of maximum opening for joint j, in
radians (1.05 for the shoulder, 1 for the elbow, 0.4
for the wrist);
amin
j
= degree of maximum closure for joint j, in
radians (0.13 for the shoulder, 0.11 for the elbow, -
0.5 for the wrist);
TheIdeomotorPrincipleSimulated-AnArtificialNeuralNetworkModelforIntentionalMovementandMotorLearning
227

k
j
= force intensity p
j,t
for the joint j, empirically
chosen (0.5 for the elbow and shoulder, 0.14 for the
wrist).
2.1.2 Neural Network
The ANN was a two-layer neural network
comprising 5 input units and 6 output units, fully
connected with anterograde connections from input
to output. There were no hidden units. The input
units were simple linear units. The output units were
classic sigmoid units, having an analogic output
ranging from 0 to 1 and equipped with modifiable
learning bias. Their output values were copied into
equation (1) (variables e
j,t
and f
j,t
for any joint j) to
compute limb movements. The first two input units
(S1 units) received “visuospatial” information on the
hand position, encoded in polar coordinates (angle in
radians with respect to the posterior-anterior axis
and distance from the shoulder, normalized for the
overall length of the fully extended limb). The last
three input units (S2 units) received
“proprioceptive” information on the opening angle
for each of the three joints, normalized between -1
and 1. Before each movement the two S1 units also
received commands from Motor Will.
2.2 Simulation Flow
When the simulation began, the connection weights
and the output unit biases were initialized with
random values ranging from -0.25 to +0.25. The arm
was positioned with all the joints partly opened.
After the initialization stage, the simulation
proceeded in turns, each turn comprising the two
phases, movement and learning, each comprising
three steps.
2.2.1 Movement
1. The input units received sensory information
from the arm: S1 units received the spatial location
of the hand, and S2 units the angles from the three
joints.
2. Motor Will overwrote S1 input unit activations
with activations corresponding to a random desired
hand position.
3. The input units activated the output units, and the
joint opening angles therefore changed according to
equations (1) and (2). The actual output values were
recorded for use in the ensuing learning phase,
during which they yielded the desired output, target
activations. The difference in pixels (spatial errors)
between the desired hand position (target position)
and the hand position actually reached, were
measured and recorded. This spatial error
measurement served only to evaluate network
performance and not to assess motor learning.
2.2.2 Learning
1. The sensory pathways conveyed to the S1 units
information on the new hand position.
2. The input units activated the output units again,
this time using the new activation values obtained
from the S1 units corresponding to the hand position
actually reached. These outputs left the joint angles
unchanged, they served only for learning. These new
outputs were the ones the network would produce if
the desired movement were actually the movement
achieved in movement phase 2.2.1, step 3. The
difference between the current outputs and the
outputs recorded in that phase was the error to
minimize during learning.
3. A standard delta rule (Rumelhart, Hinton &
Williams 1986) was applied to minimize the error
vector calculated in the former step. The results we
describe were obtained with a learning rate = 0.1 and
momentum = 0.25.
2.3 Tests
Besides evaluating the “online” spatial error after
every movement (section 2.2.1 step 3), after every
5000 movements the program temporarily stopped
the simulation, and submitted the network to an
“offline” test entailing a predefined set of 588
movements (Fig. 2A) commanded by the Motor Will
transmitting to the S1 units the polar coordinates for
the 588 successive turns. During testing, the learning
phase (section 2.2.2) was skipped. For each of the
588 test movements the position actually reached by
the hand and the corresponding spatial error were
recorded for later evaluation offline.
We used these procedures to conduct several
simulations. In some simulations we introduced a
sort of “sensory blind spot”, a wide circular area,
covering up to 50% of the workspace and
differentially positioned in the various simulations
(Fig. 3A), where we skipped the learning phase
(2.2.2) when the hand ended up in this area.
In other trials, to assess whether learning
depended on precise physical values inherent to the
system, and to verify whether the controller system
adapted to changes in the controlled system, we
varied the sensory code used for hand position or the
limb segment mass (variable m
j
in equation 1), right
from the beginning, or after advanced learning
(30000 movements).
NCTA2014-InternationalConferenceonNeuralComputationTheoryandApplications
228

Figure 2: Progressive improvement in performance during
the 588 test movements with motor experience. Small
circle = hand starting point; black points = hand
movement arrival point. A: target points; B: points
effectively reached before learning; C: after 5000 random
movements and D: after 30000 random movements.
3 RESULTS
In all the simulations the tested ANN system
improved from a mean spatial error of more than
150 pixels when simulation began to an error of less
than 15 pixels after 10,000 movements (few tens of
seconds on a modern pc) and fewer than 7 pixels
after 30000 movements. These results underwent
minimum variability owing to random elements used
for initializing weights and movement choices
decided by Motor Will.
The network’s motor performance, as assessed
with periodic testing using the 588 target points,
progressively improved with experience, improving
from a mean spatial error of about 95 pixels at turn
zero (Fig. 2B) to 53 pixels at turn 5000 (Fig. 2C),
and 13 pixels at turn 30000 (Fig. 2D) (these values
differ from those for the mean spatial error
mentioned above because they only refer to the 588
test movements instead of all movements). Before
motor learning started, the points effectively reached
clustered in an area corresponding to the
intermediate arm positions (Fig. 2B). As the network
acquired experience, arm movements gradually
expanded and after 30000 movements covered the
workspace in a fairly uniform manner (Fig. 2D)
acceptably matching the targets.
After 30000 movements, spatial error
distributions showed that the system performed well
over the whole workspace, except in the extreme tail
in the drop-shaped area corresponding to extreme
extension (Fig. 3B). The sensory blind spot had
scarce influence on learning improvements (Fig.
3C,D). These results remained uninfluenced by the
hand sensory code used, nor did they significantly
suffer from mass changes in limb segments, before
or after motor learning.
Figure 3: Spatial error distribution for the 588 test
movements after 30000 random movements with and
without no-learning areas (“sensory blind spot”). A:
workspace area (dark grey area) with a generic blind spot
(white disk); B: errors (in grey color code) without the
sensory blind spot; C, D: with the sensory blind spot
(black outline circle) in two different sizes and positions.
Values are for spatial error in pixels.
4 DISCUSSION
4.1 Comments on the Model
The simplified ANN simulation, focusing on the
basic IMP features insofar as motor commands and
sensory feedback reach the same S1 input units,
effectively learned to move the arm in the
workspace. It learned acceptably well even when we
varied influential experimental variables such as the
sensory code used for hand position, the mass for the
limb segments to move, and when the ANN was
able or unable to receive sensory feedback about
movements performed in the workspace (sensory
blind spot). Our decision to disregard velocity
sensory information or hidden neural network units
had no apparent influence on our model’s functional
ability thus confirming that these variables are
unessential to model functioning.
Our IMP model reproduces with acceptable
approximation the various human motor learning
properties, such as learning from experience, ability
to work regardless of the specific body segment
features, ability to adapt to changes in these features,
and the fact that even randomly-generated
movements contribute to learning (infantile motor
babbling). Like the human motor learning system,
TheIdeomotorPrincipleSimulated-AnArtificialNeuralNetworkModelforIntentionalMovementandMotorLearning
229

our ANN underwent completely unsupervised
learning. We never used external sample sets. The
ANN itself generated learning examples from its
random movements and errors. For learning we
never measured spatial errors between the desired
movements and those actually made. Conversely, as
the learning error we used the difference between
output unit activations in two different functional
phases (phase 2.2.1 step 3 and in phase 2.2.2 step 2),
values completely and locally available to the net.
In our proposed model the movement learned is
not the desired movement but the movement
effectively done (section 2.2.2). The system
nevertheless succeeds in performing with reasonable
precision even movements never previously done
(the 30000 learned movements taken as a typical
number of movements for simulation are less than a
mere 0.0001% of the over 300 million movements
possible in the workspace, and the test movements
described in section 2.3 and those finishing in the
sensory blind spot were even explicitly excluded
from learning). This system ability evidently stems
from an ANN’s well-known ability to generalize
(Caudill & Butler 1992), a feature allowing our
ANN to interpolate and extrapolate information
from the movements done, thus filling in unexplored
movements and forming the general sensorimotor
map valid for all movements.
Even though these model features are
biologically plausible, other features are biologically
less plausible, at least with the essential model
architecture we used. For example, in particular, the
proposed learning system requires special timing.
After the movement, when sensory feedback from
the hand position returns to the S1 units, the S2 units
must still retain information on the limb state before
the movement, and the output units must still retain
information about the activation that caused the limb
muscles to contract. Hence during learning, the
network must have constantly available all three
components mentioned in section 2.1: neuronal
activations coding the initial limb state, the final
state, and those causing the limb to pass from its
initial to its final state, that we will henceforth call
learning triplets, or simply triplets. In a computer
software algorithm this requirement poses no
problems whereas in biological nervous systems it
raises several concerns. A more realistic model to
simulate a biological motor system should therefore
include accessories such as memory units and units
that regulate activation flow to and from the
network. These accessories become even more
essential as the possible time shift between the three
triplet components increases, as it does in the
extended model we propose in the next section.
4.2 Triplet Chaining
The model we propose here extends IMP from
elementary movements to more complex behaviors
thus unifying the various intentional movement
scales under a single principle. The ANN model we
have examined so far applies to elementary
movements. Conversely, the chained triplet model
can also account for more complex actions, where it
can also provide a new insight into neuronal
populations such as canonical neurons (Shepherd
1992) and mirror neurons (Di Pellegrino et al. 1992;
Casile 2013) that have been found in biological
nervous systems and whose real function remains
debatable.
The extended triplet model that we propose here
involves several triplet-networks, linked so that the
output units for each preceding triplet-net also act as
the S1 input units for the ensuing triplet-net. The S1
and S2 input units can receive sensory information
not only from the osteo-muscular system, but from
the whole body and external environment. In this
chain, the S1 input units on the first net receive the
actions desired by Will (actions that are more
abstract than the simple and concrete desire to bring
the hand to a desired position), and the ensuing nets
progressively increase the level of detail and
concreteness for the actions needed to satisfy the
desire. Finally, the final net (the net described in the
basic model) generates the neuromuscular
activations required to perform the selected
action(s).
For example, if a person is hungry and sees an
apple at hand, the S2 units for the triplet-nets in the
chain receive this information as the actual/initial
state. If Will generates and transmits to the first net’s
S1 units the desired state “no longer hungry”, then
the first net, which has learned from experience that
when one is hungry the action for curbing hunger is
to eat, generates the sensory-coded desired action
“eat” as activations on its output units. These output
units in the first net are also the S1 input units in the
second net, so “eat” becomes the final desired state
(in this case a desired action) for the second net.
The second net has learned from experience
which objects are edible, and when the desired
action is to eat and the object is edible, it generates
on its output units the sensory-coded action “eat the
object”, which becomes the desired action for the
third net S1 units.
NCTA2014-InternationalConferenceonNeuralComputationTheoryandApplications
230

Having learned from experience that when faced
with an apple and a desire to eat it the action to eat it
is to get it, the third net now generates on its output
units the sensory-coded action “get the apple”,
which becomes the desired action for the fourth net.
“Get the apple” is still sensory encoded though,
so it actually pictures “arm extended to the apple and
fingers tightened on the apple”. This is the desired
final state for the fifth net, the net described in the
basic model, the one that also receives on its input
units sensory information about the current arm state
and activates the arm muscles.
Essentially, we suggest that in the nervous
system voluntary actions or behaviors are triggered
by formulating their end-effects as high-level
sensory representations of the desired results. These
representations are generated in the prefrontal
cortex, especially in the dorsal and lateral prefrontal
areas (Haber 2003; Watanabe & Sakagami 2007;
Tanji & Hoshi 2008). These areas act as a high-
level, ‘strategic’ Motor Will by generating sensory
representation for the desired result (goal), without
focusing on details in its execution, other than
possibly enforcing context-related constraints (e.g.
to avoid an obstacle in grasping an object).
These sensory representations consist of neuron
activation-and-inactivation combinations in the
prefrontal areas, which in turn evoke sensory
representations in the frontal premotor areas and in
parietal, occipital, and temporal sensory and
associative areas. These parietal, occipital, and
temporal areas encode both sensory-specific
representations for the goal (symbolized by the S1
units in our model) and actual sensations from the
body and the environment relevant to the task
(symbolized by the S2 units in our model). These
representations and sensations are locally sensory-
specific: tactile or proprioceptive in the parietal lobe,
visual in the occipital lobe, and acoustic or visceral
in the temporal lobe. Unlike these areas, the
premotor areas encode the goal in a more abstract
and multisensory way. Premotor area neurons are S1
units in our model. Other S1 and S2 units are
probably located in sub-cortical structures,
especially in the basal ganglia (S1 units) and the
thalamus (S2 units).
All these representations then travel throughout
these areas, converging towards the primary sensory
(S1, postcentral gyrus) and motor (M1, precentral
gyrus) areas and the sub-cortical motor structures
through subsequent elaboration steps, represented in
our model by the chained triplets that progressively
detail the appropriate elementary actions needed to
reach the goal. These representations gain motor
detail as they converge to the S1 and M1 brain areas.
Until the very last step, the first and only one that
really encodes the former sensory action
representation into the motor effector
(neuromuscular) space, all these representations are
sensory-coded. The neurons making the final
sensorimotor translation (the output group in our
basic model) are probably located in sub-cortical
motor structures, or even in the spinal chord.
This model is consistent with increasing
evidence from motor research in primates and
humans (reviews in Lebedev & Wise 2002,
Graziano 2006, Cisek & Kalaska 2010. See also
Miller 2000, Miller & Cohen 2001, Haber 2003,
Tanji & Hoshi 2008 for specific reviews on the role
of the prefrontal areas in voluntary movement;
Rizzolatti & Luppino 2001, Rozzi et al. 2008, Koch
et al. 2010 for the role of parietal areas; Burnod et
al. 1999 for flow and distribution of movement-
related sensory representations; and Zinger et al.
2013 for the functional organization of information
flow in the corticospinal pathway and joint
specificity of M1 sites). The stages progressively
elaborating and subdividing motor goals into triplet-
nets are not necessarily exactly those we describe.
What our simplified model allows us to conclude is
that the general features underlying triplet network
chaining concord well with current knowledge on
intentional movement.
Along the triplet chain, the role and function of
some known as well as elusive neuron populations
become clearer. In particular, the function of the
second network in the chain reasonably recalls
known canonical neuron properties. The function of
the third network recalls known mirror neuron
properties, at least those described for certain major
mirror neuron subpopulations, which seem
essentially to encode the subject’s ability to interact
with objects (Caggiano et al. 2009, 2011; Casile,
Caggiano & Ferrari 2011) and reasons for grasping
an object (Casile, Caggiano & Ferrari 2011). Hence
the interpretation our sensorimotor model offers for
mirror neurons is that they primarily exist to allow
us to move intentionally, being a step in
sensorimotor mapping that descends from general,
high-level sensations (“I am hungry”) and Will-
desired sensations (“no longer hungry”) to the
actions (“get the apple”) able to make the desired
sensations real. This is a more basic and critical
function than functions other explanations propose,
for example that mirror neurons are essential for
learning by imitation, for the theory of mind, or for
empathy (Gallese & Goldman 1998; Gallese 2001;
Gallese, Eagle & Migone 2007; Iacoboni 2009).
TheIdeomotorPrincipleSimulated-AnArtificialNeuralNetworkModelforIntentionalMovementandMotorLearning
231

These earlier conjectural explanations remain
unproven and highly controversial (Borg 2007;
Hickok 2009; Dinstein et al. 2010; Heyes 2010;
Decety 2011; Lamm, Decety & Singer 2011) and are
somewhat disconcerting when we consider them in
the monkey, the species in which mirror neurons
have been primarily found. Conversely, more
recently emerging findings (Caggiano et al. 2009,
2011; Casile, Caggiano & Ferrari 2011; Casile 2013)
seem in line with the model we propose, insofar as
they showed that many mirror neurons exist to
encode the subject’s interaction with objects, rather
than similar interactions by others. These “special”
mirror neurons and the classic mirror neurons that
also respond to seeing “their” action performed by
others should be considered together rather than
individually. Hypotheses considering single neurons
isolated from neuron combinations should be
regarded with caution, especially given that the only
study that demonstrated mirror neurons in man
(Mukamel et al. 2010) found confusing and even
contradictory individual neuron responses.
5 CONCLUSIONS
Our unsupervised ANN simulation confirms, as the
IMP claims, that voluntary actions can be initiated
by imagining (desiring) their sensory effects. IMP
seems a valid model for understanding human
sensorimotor mapping, intentional movement and
motor learning. Detailing and extending the IMP in
what we termed the “chained triplet-net” model
makes the IMP also helpful in explaining voluntary
behavior besides elementary actions. Along this
chain, elusive neuronal systems such as the
canonical and mirror neuron systems acquire
definite meanings. Future research should endeavor
to identify which other non-motor nervous functions,
such as cognitive functions, the extended IMP and
the triplet model might help to explain.
ACKNOWLEDGEMENTS
We thank Miss Alice M. Crossman for her
contribution in revising the text.
This work received financial support from
Castello della Quiete Srl (Rome, Italy).
REFERENCES
Borg, E., 2007. If mirror neurons are the answer, what was
the question? Journal of Consciousness Studies, 14(8),
5-19.
Burnod, Y., Baraduc, P., Battaglia-Mayer, A., Guigon, E.,
Koechlin, E., Ferraina, S., Lacquaniti, F. & Caminiti,
R., 1999. Parieto-frontal coding of reaching: an
integrated framework. Experimental Brain Research,
129(3), 325-346.
Butz, M. V., Herbort, O. & Hoffmann, J., 2007. Exploiting
redundancy for flexible behavior: unsupervised
learning in a modular sensorimotor control
architecture. Psychological Review, 114(4), 1015.
Caggiano V., Fogassi L., Rizzolatti G., Thier P. & Casile
A. 2009. Mirror neurons differentially encode the
peripersonal and extrapersonal space of monkeys.
Science, 324(5925), 403–406.
Caggiano V., Fogassi L., Rizzolatti G., Thier P., Giese
M.A. & Casile A., 2011. View-based encoding of
actions in mirror neurons in area F5 in macaque
premotor cortex. Current Biology, 21, 144–148.
Casile, A., Caggiano, V. & Ferrari, P. F., 2011. The mirror
neuron system a fresh view. The Neuroscientist, 17(5),
524-538.
Casile, A., 2013. Mirror neurons (and beyond) in the
macaque brain: an overview of 20 years of research.
Neuroscience Letters, 540, 3-14.
Caudill, M. & Butler, C., 1992. Naturally Intelligent
Systems, MIT press.
Cisek, P. & Kalaska, J. F., 2010. Neural mechanisms for
interacting with a world full of action choices. Annual
Review of Neuroscience, 33, 269-298.
Dayan, E., Casile, A., Levit-Binnun, N., Giese, M. A.,
Hendler, T. & Flash, T., 2007. Neural representations
of kinematic laws of motion: evidence for action-
perception coupling. Proceedings of the National
Academy of Sciences, 104(51), 20582-20587.
Decety, J., 2011. Dissecting the neural mechanisms
mediating empathy. Emotion Review, 3(1), 92-108.
Di Pellegrino, G., Fadiga, L., Fogassi, L., Gallese, V. &
Rizzolatti, G., 1992. Understanding motor events: a
neurophysiological study. Experimental Brain
Research, 91(1), 176-180.
Dinstein, I., Thomas, C., Humphreys, K., Minshew, N.,
Behrmann, M. & Heeger, D. J., 2010. Normal
movement selectivity in autism. Neuron, 66(3), 461-
469.
Flanagan, J. R. & Rao, A. K., 1995. Trajectory adaptation
to a nonlinear visuomotor transformation: evidence of
motion planning in visually perceived space. Journal
of Neurophysiology, 74(5).
Gallese, V. & Goldman, A., 1998. Mirror neurons and the
simulation theory of mind-reading. Trends in
Cognitive Sciences, 2(12), 493-501.
Gallese, V., 2001. The 'shared manifold' hypothesis. From
mirror neurons to empathy. Journal of Consciousness
Studies, 8(5-7), 5-7.
Gallese, V., Eagle, M. N. & Migone, P., 2007. Intentional
attunement: Mirror neurons and the neural
NCTA2014-InternationalConferenceonNeuralComputationTheoryandApplications
232

underpinnings of interpersonal relations. Journal of
the American Psychoanalytic Association, 55(1), 131-
175.
Galtier, M., 2014. Ideomotor feedback control in a
recurrent neural network. (Online)
http://arxiv.org/pdf/1402.3563v3
Graziano, M., 2006. The organization of behavioral
repertoire in motor cortex. Annual Review of
Neuroscience, 29, 105-134.
Haber, S. N., 2003. The primate basal ganglia: parallel and
integrative networks. Journal of Chemical
Neuroanatomy, 26(4), 317-330.
Heyes, C., 2010. Mesmerising mirror neurons.
Neuroimage, 51(2), 789-791.
Hickok, G., 2009. Eight problems for the mirror neuron
theory of action understanding in monkeys and
humans Journal of Cognitive Neuroscience, 21(7),
1229-1243.
Hommel, B., 2013. Ideomotor action control: on the
perceptual grounding of voluntary actions and agents.
Action Science: Foundations of an Emerging
Discipline, 113-136.
Iacoboni, M., 2009. Imitation, empathy, and mirror
neurons. Annual Review of Psychology, 60, 653-670.
Karniel, A. & Inbar, G. F., 1997. A model for learning
human reaching movements. Biological Cybernetics,
77(3), 173-183.
Kiesel, A. & Hoffmann, J., 2004. Variable action effects:
Response control by context-specific effect
anticipations. Psychological Research, 68(2-3), 155-
162.
Koch, G., Cercignani, M., Pecchioli, C., Versace, V.,
Oliveri, M., Caltagirone, C., Rothwell, J. & Bozzali,
M., 2010. In vivo definition of parieto-motor
connections involved in planning of grasping
movements. Neuroimage, 51(1), 300-312.
Lamm, C., Decety, J. & Singer, T., 2011. Meta-analytic
evidence for common and distinct neural networks
associated with directly experienced pain and empathy
for pain. Neuroimage, 54(3), 2492-2502.
Lebedev, M. A. & Wise, S. P., 2002. Insights into seeing
and grasping: distinguishing the neural correlates of
perception and action. Behavioral and Cognitive
Neuroscience Reviews, 1(2), 108-129.
Melcher, T., Weidema, M., Eenshuistra, R. M., Hommel,
B. & Gruber, O., 2008. The neural substrate of the
ideomotor principle: An event-related fMRI analysis.
Neuroimage, 39(3), 1274-1288.
Melcher, T., Winter, D., Hommel, B., Pfister, R., Dechent,
P. & Gruber, O., 2013. The neural substrate of the
ideomotor principle revisited: evidence for
asymmetries in action-effect learning. Neuroscience,
231, 13-27.
Miller, E. K., 2000. The prefontral cortex and cognitive
control. Nature Reviews Neuroscience, 1(1), 59-65.
Miller, E. K. & Cohen, J. D., 2001. An integrative theory
of prefrontal cortex function. Annual Review of
Neuroscience, 24(1), 167-202.
Mukamel, R., Ekstrom, A. D., Kaplan, J., Iacoboni, M. &
Fried, I., 2010. Single-neuron responses in humans
during execution and observation of actions. Current
Biology, 20(8), 750-756.
Pfister, R., Melcher, T., Kiesel, A., Dechent, P. & Gruber,
O., 2014. Neural correlates of ideomotor effect
anticipations. Neuroscience, 259, 164-171.
Rizzolatti, G. & Luppino, G., 2001. The cortical motor
system. Neuron, 31(6), 889-901.
Rozzi, S., Ferrari, P. F., Bonini, L., Rizzolatti, G. &
Fogassi, L., 2008. Functional organization of inferior
parietal lobule convexity in the macaque monkey:
electrophysiological characterization of motor, sensory
and mirror responses and their correlation with
cytoarchitectonic areas. European Journal of
Neuroscience, 28(8), 1569-1588.
Rumelhart, D. E., Hinton, G. E. & Williams, R. J., 1986.
Learning Internal Representations by Error
Propagation. In Rumelhart, D. E., McClelland, J. L. &
The PDP Research Group, Parallel Distributed
Processing. Explorations in the Microstructure of
Cognition, MIT Press.
Sauser, E. L. & Billard, A. G., 2006. Parallel and
distributed neural models of the ideomotor principle:
An investigation of imitative cortical pathways.
Neural Networks, 19(3), 285-298.
Shadmehr, R., 2005. The computational neurobiology of
reaching and pointing: a foundation for motor
learning. MIT press.
Shepherd, G. M., 1992. Canonical neurons and their
computational organization. In Single Neuron
Computation. Academic Press Professional, Inc., 27-
60
Shin, Y. K., Proctor, R. W. & Capaldi, E. J., 2010. A
review of contemporary ideomotor theory.
Psychological Bulletin, 136(6), 943-974.
Stock, A. & Stock, C., 2004. A short history of ideo-motor
action. Psychological Research, 68(2-3), 176-188.
Tanji, J. & Hoshi, E., 2008. Role of the lateral prefrontal
cortex in executive behavioral control. Physiological
Reviews
, 88(1), 37-57.
Watanabe, M. & Sakagami, M., 2007. Integration of
cognitive and motivational context information in the
primate prefrontal cortex. Cerebral Cortex, 17(suppl
1), 101-109.
Wulf, G. & Prinz, W., 2001. Directing attention to
movement effects enhances learning: A review.
Psychonomic Bulletin & Review, 8(4), 648-660.
Wohlschläger, A., Gattis, M. & Bekkering, H., 2003.
Action generation and action perception in imitation:
an instance of the ideomotor principle. Philosophical
Transactions of the Royal Society of London. Series B:
Biological Sciences, 358(1431), 501-515.
Zinger, N., Harel, R., Gabler, S., Israel, Z. & Prut, Y.,
2013. Functional Organization of Information Flow in
the Corticospinal Pathway. The Journal of
Neuroscience, 33(3), 1190-1197.
TheIdeomotorPrincipleSimulated-AnArtificialNeuralNetworkModelforIntentionalMovementandMotorLearning
233
