
Preparation of a PLGA/Calcium Silicate Composite with Gradient
Pore Structure
Bong-Kyu Choi
1
, Suk Young Kim
2
and Sang-Hoon Rhee
3
1
Department of Oral Microbiology and Immunology, Dental Research Institute and BK21 HLS, School of Dentistry,
Seoul National University, Seoul, Korea
2
School of Materials Science & Engineering, Yeungnam University, Gyeongsan, Korea
3
Department of Dental Biomaterials Science, Dental Research Institute and BK21 HLS, School of Dentistry,
Seoul National University, Seoul, Korea
Keywords: Composite, PLGA, Calcium Silicate, Pore, Bioactivity.
Abstract: The PLGA/SiO
2
-CaO composite, which have a gradient pore structure, was newly prepared by the
expansion of carbon dioxide gas in the PLGA matrix. The bioactive SiO
2
-CaO particles were made by a sol-
gel method from tetraethyl orthosilicate and calcium nitrate tetrahydrate under acidic condition followed by
the heat treatment at 600
o
C for 2 h. The PLGA/SiO
2
-CaO composite was then prepared by a solvent casting
using chloroform as solvent. The composite was loaded into the high pressure chamber and then carbon
dioxide gas was introduced achieving a final pressure of 10 MPa. After 3 days, the gas was released quickly
and the gradient pore structure was developed. The samples were observed by FE-SEM and its bioactivity
was tested in simulated body fluid.
1 INTRODUCTION
The representative bone bonding materials are
calcium phosphates, bioactive-glasses, and –glass
ceramics. They are known to bond to bone directly
without intervening fibrous tissues. However, their
applications are only limited to non-load beading
sites because their fracture toughness are very low,
which results in low mechanical reliability.
Composites have been extensively studied as
alternations of a bioactive ceramic because a
polymer can give a ductility and flexibility while a
ceramic can give hardness, strength, and bioactivity.
One method to make a bioactive composite is just
mixing the ceramic particles and polymers
mechanically. Typical ones are thermal blending and
solvent casting methods. However, the drawback of
thermal blending method is that it is hard to increase
the ceramic portion in the composites as well as the
occurrence of phase separations due to the different
wettabilities between two materials. Ceramics show
hydrophilicity while most synthetic polymers have
hydrophobicity so they cannot mix together easily.
Solvent casting method is very simple way to make
ceramic/polymer composite but it also shows the
disadvantage when mixing two phases. After casting
the ceramic/polymer mixture, the sedimentation of
ceramic particles must occur due to the density
differences between solvent and ceramic particles.
Thus, thick polymer top layer generally forms. In
addition, phase separation between ceramic particles
and polymer matrix occurs after drying. Thus,
ceramic/polymer nano-composites are developed,
where the polymer is linked to ceramic precursor at
the molecular lever.
However, the reported process to make the
composite is hard to get porous structure. The bone
grafting materials must have porous structure
because it induces the growth of blood vessels into
the material.
The salt leaching method is generally used to make
porous structure when using the solvent casting
method to make ceramic/polymer composites.
However, this method is hard to make the
connections among the pores. In addition, it cannot
be applicable to the process for making nano-
composite because the calcium salt, which is
inevitable component to produce bioactivity in vivo,
is also leached out when washing out the salt after
the casting.
Mooney et al. (1996) reported the new method to
produce porous structure in PLGA scaffold by the
239
Choi B., Young Kim S. and Rhee S..
Preparation of a PLGA/Calcium Silicate Composite with Gradient Pore Structure.
DOI: 10.5220/0004200302390242
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2013), pages 239-242
ISBN: 978-989-8565-34-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

expansion of carbon dioxide gas in high pressure
chamber. The carbon dioxide has some solubility in
the PLGA. Thus, when the high pressure of carbon
dioxide is applied to the PLGA scaffold and then
released quickly, it becomes to expand in the PLGA
matrix. Resultantly, the pores are developed at the
places where the gas existed. However, the
disadvantage of this method is that it is also hard to
connect between the pores. Thus, the salt leaching
method is combined together to make porous
structure. However, there has been no reprot to
apply this method to make a porous bioactive
composite material, yet.
In this study, we prepare the PLGA/calcium
silicate composite by the solvent casting method and
then a gradient pore structure was introduced using
the expansion of carbon dioxide gas in a high
pressure chamber.
2 MATERIALS AND METHODS
2.1 Preparation of Calcium Silicate
Powders
The calcium silicate (SiO
2
-CaO) particles were
prepared with the starting composition of
70SiO
2
30CaO in molar ratio. The calcium silicate
particles were prepared by hydrolysis and
polycondensation of tetraethyl orthosilicate (TEOS,
Nacalai Tesque) in calcium nitrate tetrahydrate
(Nacalai Tesque) and polyethylene glycol (PEG,
Aldrich) in aqueous solution. The molecular weight
of PEG used in this experiment was about 10000.
PEG and calcium nitrate tetrahydrate were
dissolved in distilled water and then concentrated
nitric acid (60 wt%, Nacalai Tesque) was added.
TEOS was added to the above solution under
stirring. After 20 min, the solution was transferred to
a polystyrene box with its top sealed tightly, and
kept at 40C in a convection oven for gelation and
aging for 1 day. The obtained wet gel was immersed
in distilled water for 3 h with the distilled water
renewed every hour. After the wet gel was dried at
40C for 7 days, it was heated at 600C for 2 h and
then pulverized using a planetary ball mill.
2.2 Preparation of a PLGA/Calcium
Silicate Composite
The 90PLGA/10SiO
2
-CaO composite (in wt%) was
made by the solvent casting. PLGA powder
(90PLA10PGA, IV 0.55 ~ 0.75, Medisorb,
Alkemes) was dissolved in chloroform (4%) after
which SiO
2
-CaO particles were added to the
solution. After stirring vigorously for 1 h, the
mixture was poured into a Teflon mould and dried
under ambient conditions.
2.3 Preparation of a PLGA/Calcium
Silicate Composite with a Gradient
Pore Structure
The 90PLGA/10SiO
2
-CaO composite was loaded
into the high carbon dioxide pressure chamber and
then carbon dioxide gas was introduced into the
chamber achieving final pressure of 10 MPa. The
specimens were allowed to equilibrate and saturate
with the carbon dioxide gas for 3 days, forming a
single phase PLGA/CaO-SiO
2
/CO
2
gas solution at
room temperature. Subsequently, the carbon dioxide
gas was quickly released bringing the chamber to
ambient pressure. (Mooney et al., 1996)
2.4 Bioactivity Test
The bioactivity of the PLGA/CaO-SiO
2
composite
was assessed by evaluating its capability to form low
crystalline hydroxyl carbonate apatite on its surface
in simulated body fluid (SBF). (Kokubo et al., 1990)
The SBF was prepared by dissolving reagent grade
NaCl, NaHCO
3
, KCl, K
2
HPO
4
·3H
2
O, MgCl
2
·6H
2
O,
CaCl
2
, and Na
2
SO
4
in ion exchanged distilled water.
Their ionic concentrations were Na
+
142, K
+
5.0,
Mg
2+
1.5, Ca
2+
2.5, Cl
-
147.8, HCO
3
-
4.2, HPO
4
2-
1.0, SO
4
2-
0.5 (in mM). The solution was buffered at
pH 7.4 with tris(hydroxymethyl) aminomethane
((CH
2
OH)
3
CNH
2
) and 1 M hydrochloric acid (HCl)
at 36.5
o
C.
Specimen disks 12 mm in diameter by 4
mm in thickness were cut, sterilized under UV lamp
for 30 minutes, and then dried on a clean bench.
Subsequently, the specimens were incubated in 30
mL of the SBF at 36.5
o
C for 7 days. After
incubation, the PLGA/CaO-SiO
2
composite was
removed, gently rinsed with ion-exchanged distilled
water several times, and dried at room temperature.
2.5 Characterization
Microstructure was observed by a field emission
scanning electron microscopy (FE-SEM; S-4700,
Hitachi). The crystal phase of the specimens before
and after soaking in the SBF was evaluated by a thin
film X-ray diffractometry (TF-XRD; D8 Discover,
Bruker).
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
240
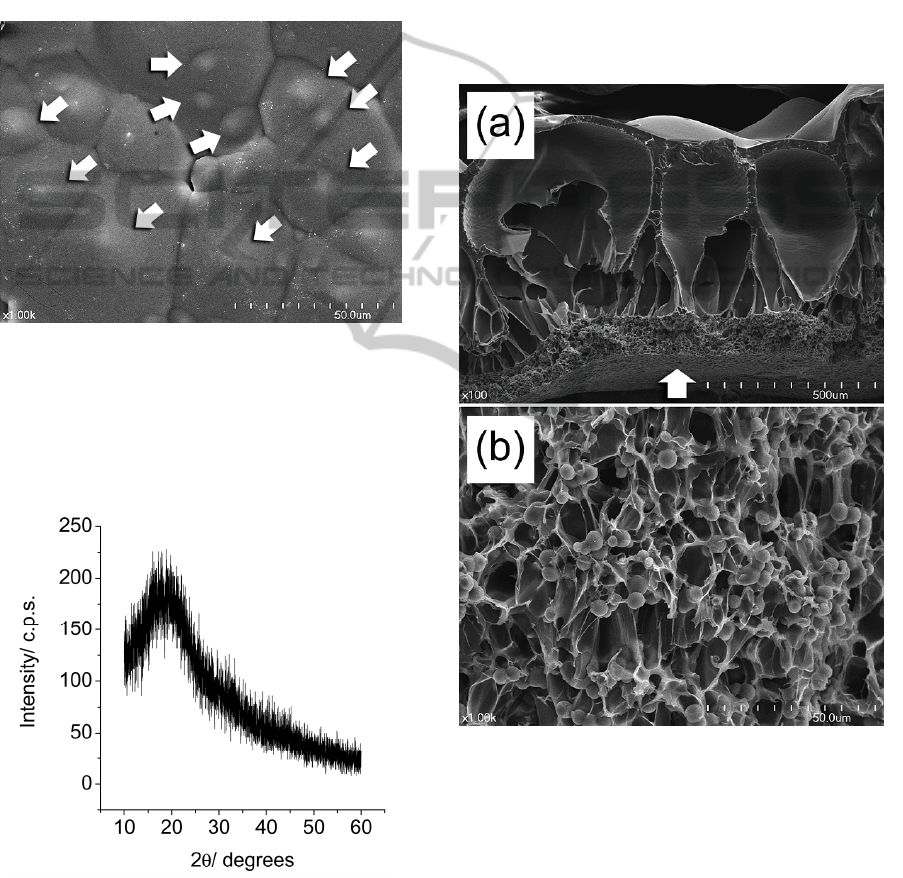
3 RESULTS AND DISCUSSION
Figure 1 shows the FE-SEM photograph of the
PLGA/CaO-SiO
2
composite after the solvent casting.
A thick surface layer was the PLGA while the CaO-
SiO
2
particles were not observed at the surface.
However, the hemispherical convex surfaces
denoted as white arrows in Figure 1 must be the
CaO-SiO
2
particles, which placed under the thick
PLGA film.
Figure 1: FE-SEM photograph of the PLGA/CaO-SiO
2
composite after the solvent casting.
Figure 2 shows the XRD diffraction pattern of
the PLGA/CaO-SiO
2
composite after the solvent
casting. Only broad hallow peak was observed to
occur. It means there is no crystalline phase.
Figure 2: The XRD diffraction pattern of the PLGA/CaO-
SiO
2
composite after the solvent casting.
Figure 3 shows the (a) low and (b) high
magnification FE-SEM photographs of the fractured
PLGA/CaO-SiO
2
composite after the carbon dioxide
gas treatment. The thick PLGA surface layer was
inflated in large degree after the quick release of
carbon dioxide gas and resultantly it made large
pores at the surface of the composite (Figure 1(a)).
However, the PLGA/CaO-SiO
2
composite region,
which was previously placed under the thick PLGA
layer, was not inflated as much as the PLGA. Thus,
only small pores were produced (about 10 ~ 30 m
in size) (Figure 3(b)). The pore sizes were gradually
become larger from the bottom to top surface of the
specimen. The bottom layer of the composite
showed open pore structure (white arrow in Figure
3(a)).
Figure 3: (a) Low and (b) high magnification FE-SEM
photographs of the fractured PLGA/CaO-SiO
2
composite
after the carbon dioxide gas treatment.
Figure 4 shows the (a) low and (b) high
magnification FE-SEM photographs of the
PLGA/CaO-SiO
2
composite after soaking in the SBF
for 1 week at 36.5
o
C. Small flake-like low
crystalline hydroxyl carbonate apatite crystals were
observed to occur on the surface of the PLGA/CaO-
SiO
2
composite. They started to occur from the
surface of the CaO-SiO
2
particles and then spread
PreparationofaPLGA/CalciumSilicateCompositewithGradientPoreStructure
241
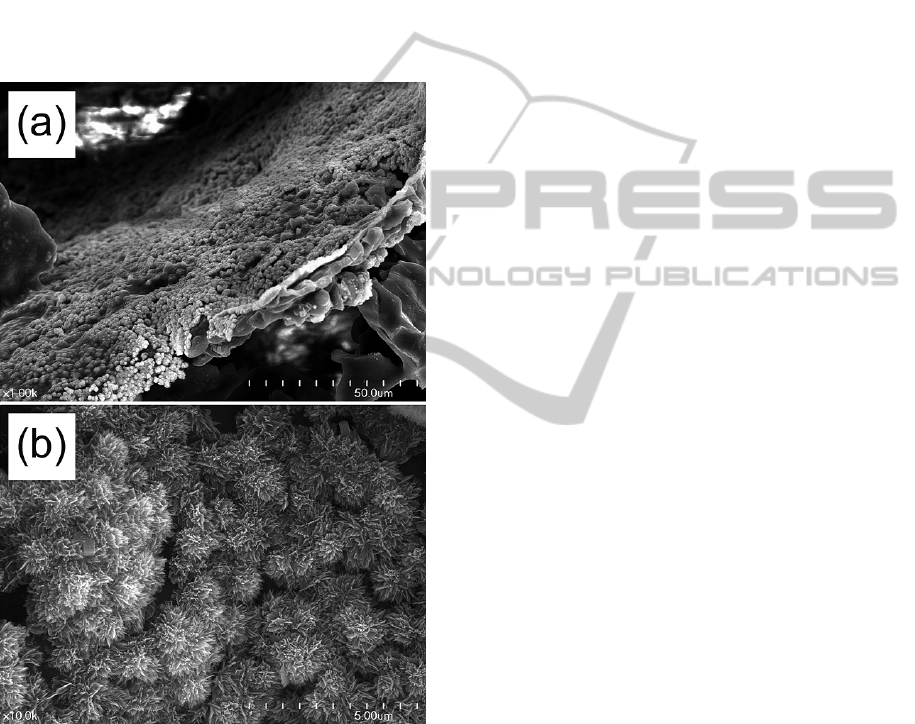
out all the surface of the composite even on the
PLGA surface. It means this PLGA/CaO-SiO
2
composite has bioactivity and it also has a potential
to have a good osteoconductivity.
From the results, it can be summarized that the
PLGA-calcium silicate composite which had the
gradient pore structure was successfully made using
the expansion of carbon dioxide gas in the high
pressure carbon dioxide gas chamber. The practical
implication of this result is that this material can be
used as a bone grafting material or a scaffold
material for bone tissue engineering due to its high
porosity and apatite forming capacity in the SBF.
Figure 4: (a) Low and (b) high magnification FE-SEM
photographs of the PLGA/CaO-SiO
2
composite after
soaking in the SBF for 1 week at 36.5
o
C.
4 CONCLUSIONS
The PLGA/calcium silicate composite which had
gradient pore structure was newly developed. The
calcium silicate particles could be made by sol-gel
method while the PLGA/calcium silicate composite
could be made by solvent casting method. The thick
PLGA layer was formed during the casting process
due to the sedimentation of the calcium silicate
particles by the different densities between PLGA
and calcium silicate particles. After the treatment of
carbon dioxide gas, the gradient pore structure was
developed in the composite. This composite showed
the bioactivity in the SBF and it means it has a high
potential to be used as a bone grafting material or
the scaffold materials for bone tissue engineering.
ACKNOWLEDGEMENTS
This research was supported by the Bio & Medical
Technology Development Program of the National
Research Foundation funded by the Korean
government (MEST) (20110007746).
REFERENCES
Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T.,
1990. J. Biomed. Mater. Res., 24, 721-734.
Mooney, D. J., Baldwin, D. F., Suh, N. P., Vacanti, J. P.,
Langer, R., 1996. Biomaterials, 17, 1417-1422.
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
242
