
Leveraging an Electronic Medical Record to Improve Compliance
with Pediatric Asthma Care Documentation
James A. Menke and Jeffery Hoffman
Nationwide Children’s Hospital, The Ohio State University, 700 Children’s Drive, Columbus, OH, U.S.A.
Keywords: Asthma Care, Compliance, Quality Improvement, Electronic Medical Record, Pediatrics.
Abstract: Asthma is our institution’s third most common admitting diagnosis with 653 admissions in 2011. The Joint
Commission monitors core measures of pediatric asthma care during hospitalization: (1) were relievers
given (2) were systemic corticosteroids given and (3) was the patient discharged with a complete asthma
action plan (AAP). We describe the sue of three standard quality improvement (QIP) cycles to improve
compliance using a computerized AAP. Our historical compliance using paper documentation averaged
32%. In Phase 1, we replaced the paper AAP form with an electronic version within our Electronic Medical
Record (EMR) and improved our average compliance to 45%. In Phase 2, we identified barriers to
additional improvement and modified the electronic form with soft stops and visual reminders. These
modifications improved our compliance to 70%. In Phase 3, we identified remaining barriers, modified the
form to include automated decision support and defaulting and improved our compliance to 90%. Using this
phased QIP, we were able to achieve significant improvement in overall compliance with the core measure
of providing an accurate and complete asthma action plan at the time of hospital discharge. With additional
QIP cycles, we believe achievement of 100% documentation compliance for this core measure is possible.
1 INTRODUCTION
The Electronic Medical Record (EMR) provides a
number of advantages over the paper record which
include: 1) access to the patient record by multiple
providers at multiple locations simultaneously; 2)
elimination of transcription errors when using
computerized order entry (COE) and other
automated entry; 4) automation of tasks; 5)
automation of calculations; 6) automatic dose
checking; 7) automatic allergy and drug interaction
checking; and 8) automatic alerting to providers for
safety, compliance and best practice issues
(Chaudhry, 2006).
Since asthma is a common reason for admission
to hospital, the Joint Commission monitors three
core measures of pediatric asthma care to assess
quality of care during a hospital stay. These
measures are: (1) were relievers given, (2) were
systemic corticosteroids given, and (3) was the
patient discharged with an accurate and complete
asthma action plan (AAP). We took take advantage
of several of these features of the EMR and created
and electronic version of our Asthma Action Plan
(core measure 3). The plan would be available in
multiple sites, viewable by multiple people
simultaneously, and editable by only one provider at
a time. This paper describes the effect on core
measure 3 compliance created by the transition from
a paper asthma action plan to one created by our
EMR using a standard quality improvement cycle
(Lodgaard and Aasland, 2011).
2 METHODS
2.1 Joint Commission Asthma Core
Measure 3
The Joint Commission core measure requires that a
home management plan of care (Asthma Action
Plan) document be given to patient/caregiver upon
discharge from the hospital. It is measured by an
audit on all patients admitted to the hospital with the
discharge diagnosis of asthma. The audit evaluates
whether a complete action plan was present in the
chart and a copy was given to the patient/caregiver.
The presence of an asthma action plan does not
necessarily reduce re-admissions, but it is evidence
of patient/family education (Morse, 2011).
319
A. Menke J. and Hoffman J..
Leveraging an Electronic Medical Record to Improve Compliance with Pediatric Asthma Care Documentation.
DOI: 10.5220/0004190403190322
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2013), pages 319-322
ISBN: 978-989-8565-37-2
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
2.2 Population
Our population consisted of all patients, age 2
through 17 years discharged to home from
Nationwide Children’s Hospital (NCH) with the
diagnosis of asthma. Excluded population – none.
2.3 Compliance Determination
and Calculation
Compliance was determined by examining the
patient chart for evidence of an asthma action plan.
To be compliant the following items must be
complete: 1) the form must be present in the medical
record; 1) the form must be specific for each patient;
4) the form must be specific for each admission; 5)
the form must be a stand-alone document; and 6)
there need to be documentation that the form was
given to the patient/family at discharge. In addition,
the following items must be contained in the action
plan: 1) asthma type and triggers; 2) reliever and
controller medications including drug name, dose,
frequency, and route; 3) follow-up medical contact
name and phone number; and 4) follow-up
information with the date and time of appointment
(or time frame). All elements must be present for the
plan to be considered compliant. There is no partial
credit. Compliance was measured quarterly.
2.4 Evaluation of Electronic Version
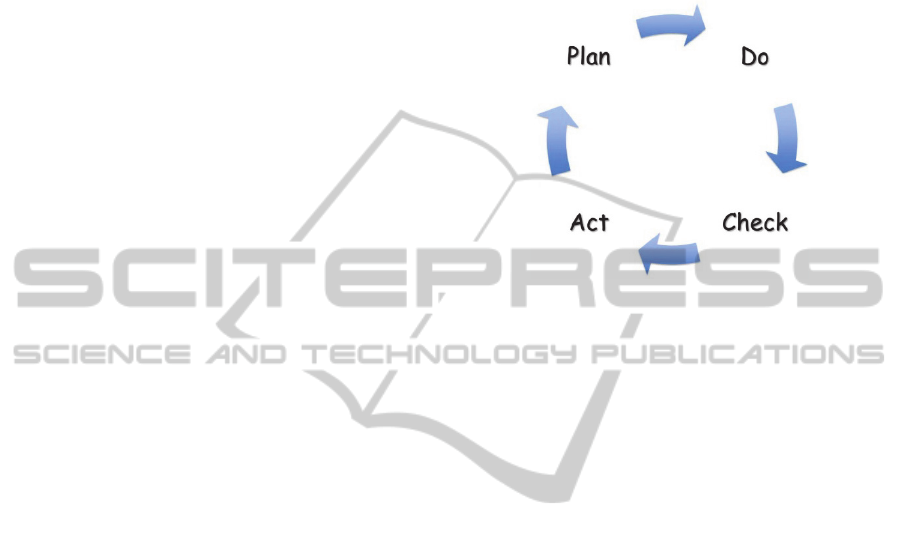
We used the Plan-Do-Check-Act (PDCA) (Fig.1)
Quality Improvement tool to evaluate and enhance
the form. The process has uses four sequential
procedures that build one upon the other. The first
procedure is Plan - where an opportunity to improve
is identified and plan a change is created. The
second procedure is Do – the implementation phase
of the process. The third procedure is Check – the
analytical phase where the implementation is
analysed and lessons about the implementation are
learned. The fourth procedure is ACT – which
produces a response to what was learned in the
check procedure. Our study applied this process to
the problem of Asthma Action Compliance and
utilized three iterations of the process. (Gabor, 1990)
2.5 Iteration 1
Plan – Identified the barriers to compliance with the
paper asthma action plan form and determined the
requirements of the electronic version of the form.
Do – Designed the electronic version of the form,
educated our users, and implemented it in our EMR.
Check – After several quarters of use, compliance
was re-evaluated and users were queried concerning
the usefulness of the electronic form and barriers to
completion. Act – Determined what modifications
were necessary to address these barriers to full
compliance.
Figure 1: The Plan Do Check Act Cycle.
2.6 Iteration 2
Plan – Designed modifications to form in an effort to
further improve compliance. Do – Implemented the
modified form in our EMR and further educated the
users. Check – After several quarters of use,
compliance was re-evaluated and the users were
queried concerning the usefulness of the electronic
form and barriers to completion. Act – Determined
what modifications were necessary to address these
barriers to full compliance.
2.7 Iteration 3
Plan – Designed modifications to form in an effort to
further improve compliance. Do – Implemented the
modified form in our EMR and further educated the
users. Check – After several quarters of use,
compliance was re-evaluated and the users were
queried concerning the usefulness of the electronic
form and barriers to completion. Act – Determined
what modifications were necessary to address these
barriers to full compliance.
2.8 EMR Software and Toolkit
Our EMR software is EpicCare Inpatient/EpicCare
Ambulatory supplied by Epic Systems Inc. (Verona,
WI). Our medical informatics team created the
Asthma Action Plan using a built-in form designer
that allowed for What-You-See-Is-What-You-Get
(WYSIWYG) design and discrete data storage for
reporting and clinical decision support. The features
HEALTHINF2013-InternationalConferenceonHealthInformatics
320
of the form that we needed for success was: 1) fully
compliant with Joint Commission standards; 2) easy
to create, edit, and maintain; 3) easy to read, full-
color, and a single page; 4) easy for anyone to view
and print; 5) easy to access reference materials; 6)
easy to access home medication lists; and 7) easy to
access a complete audit trail of the form
3 RESULTS
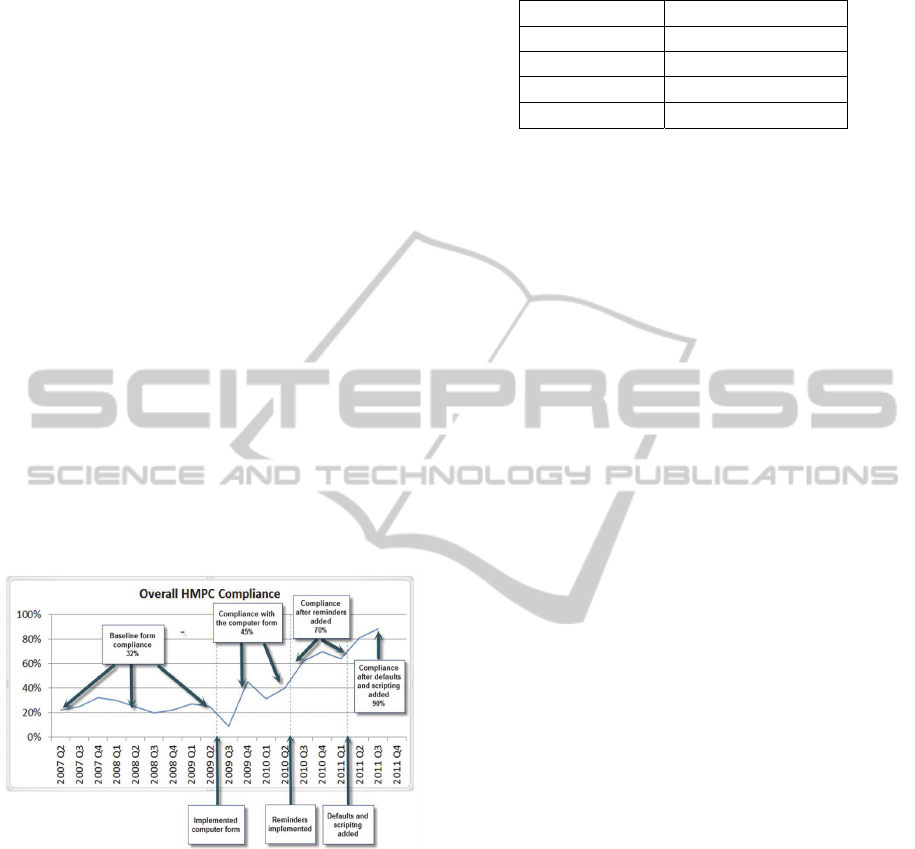
Our compliance data is shown in figure 2 and Table
1. Our highest baseline compliance using the paper
asthma action plan was 32%. The electronic asthma
action plan contained pick lists and check boxes for
much of the data entry. It also contained hyperlinks
to NIH asthma guidelines and a link to display the
patient’s current medication list present in the EMR.
After the electronic asthma action plan was
implemented, our compliance increased to 45%.
Upon reviewing the compliance data, we noted
continued deficiencies in the following required
documentation fields: 1) asthma type, 2) asthma
triggers, 3) the name and phone number of the
patient’s primary care physician when that provider
Figure 2: Compliance Over Time.
was not present within our existing database, and 4)
the specific date and time (or time frame) of
discharge follow-up. We added visible
recommendation flags to the areas of the form where
these items are entered. Users were educated and the
revised form was implemented. Compliance
following these changes increased from 45% to 70%
with these modifications. Our compliance data is
shown in figure 2.
After evaluating the compliance data following the
second iteration and receiving input from the users,
we identified additional barriers to compliance. This
included continued poor documentation of inpatient
Table 1: Compliance changes through 3 cycles.
Compliance
Pre QI 32%
After Iteration 1 45%
After Iteration 2 70%
After Iteration 3 90%
follow up information, placing inpatient follow up
information in the outpatient forms, and complaints
by users about repetitive actions. To address these
issues, we enabled functionality with the form to
default a standard post-discharge follow up time
frame if not specified, to hide the inpatient follow up
section of the form entirely when accessed from an
outpatient encounter, and created quick macro
buttons to populate common combinations of
medication therapies for user convenience.
Following the introduction of these changes,
compliance rose again from 70% to 90%.
4 DISCUSSION
Based on our experience, transitioning to an
electronic asthma action plan provides for
functionality and access that improves overall
compliance. However, merely the implementation of
an electronic equivalent of the paper form within our
EMR was not sufficient to substantially improve
compliance. According to the users, the electronic
form was somewhat easier to use than the paper
version and was more readily available, but still
suffered limitations and barriers to regular
completion. It was easier to use because it contained
pick lists for rapid documentation of items such has
asthma type, asthma triggers, and medications
including dose, route and frequency. It also
contained useful links including one to the NIH
guideless for asthma care and one to the patient’s
current medication list. However, these features
only increased overall compliance by 13%.
Part of the PDCA cycle is to Check (evaluate
what happened). Along with our audit, we discussed
with the users what they found helpful with the
electronic version and what improvements they
would fine useful. With this feedback, we added
additional functionality in the form of visual
indicators to direct the user’s attention to items often
overlooked. The reminders did improve the
compliance by 20%. However, we still were only
getting the forms fully completed 70% of the time as
indicated by our audit following the introduction of
this additional functionality.
LeveraginganElectronicMedicalRecordtoImproveCompliancewithPediatricAsthmaCareDocumentation
321

Using additional audits and further discussions
with the users, we were able to identify items on the
form that were still not being documented
consistently and identified the remaining barriers to
their completion. With this information, we added
additional functionality in the form of automated
decision support and content defaulting, which
would add information automatically to selected
fields if the user did not address them specifically.
For example, the form was programmed to
recognize when it was being accessed within an
outpatient encounter and automatically hid the
inpatient follow up section in that situation. Making
the form function appropriately depending on
whether patient encounter was in an inpatient or an
outpatient setting decreased user frustration in
having to ignore a section that was not relevant in
that context. In addition, the form was programmed
to default a standard inpatient post-discharge follow-
up time interval of three days if the user failed to
specify a specific date and time for the appointment.
We also added quick macro buttons for users to
quickly populate standard medication details, such
as the standard treatment for exercised-induced
asthma, with a single click. These eliminated errors
introduced by manual entry and made the form
easier and faster to complete, thus greatly improving
user satisfaction and ultimately compliance.
We found success by using a combination of the
PDCA quality improvement tool, by having the form
developed primarily by clinicians within our medical
informatics group to avoid the iterative steps
necessary when users work directly with non-
clinical analysts, and by involving our users in the
‘Check’ phase of the cycle. Also, repeating the
PDCA cycle multiple times allowed us to refine the
form rapidly so that it better met the users’ needs
and our ultimate goal of compliance. We are
currently in our fourth PDCA cycle and are
addressing the following issues: 1) additional
programming to check for missing data; 2) real time
reminders to update asthma action plan while
discharging the patient; and 3) linking the printing of
the patient’s discharge instructions with the printing
of the patient’s updated asthma action plan.
In summary, by transitioning our paper asthma
action plan to a computerized version and by using
repeated cycles of the PDCA tool, were able to
improve documentation compliance from 32% to
90%.
REFERENCES
Chaudhry B., et al. (2006). Systematic Review: Impact of
Health Information Technology on Quality,
Efficiency, and Costs of Medical Care. Annals of
Internal Medicine.
Lodgaard, E. ; Aasland, K. E. (2011). An examination of
the application of Plan-Do-Act cycle in product
development. In Proceedings of the 18th International
Conference on Engineering Design, section: Design
Methods and Tools Part 2 editor: Culley, S.J.; Hicks,
B. J.; McAloone, T. C.; Howard, T. J. & Dong, A.
Morse R. B. et al. (2011). Hospital-level compliance with
asthma care quality measures at children's hospitals
and subsequent asthma-related outcomes. JAMA
Gabor, A. The Man Who Discovered Quality, Penguin
Books, 1990.
HEALTHINF2013-InternationalConferenceonHealthInformatics
322
