
SIN
X
/SIO
2
STACKED SENSITIVE THIN FILM
FOR ISFET-BASED CHEMICAL AND BIOCHEMICAL SENSORS
Preparation and Characterization of the Stacked Thin Films and Sensors
J. F. Souza
1,2,3
, M. B. Lima
4
, I. Doi
1,2
, P. J. Tatsch
1,2
, J. A. Diniz
1,2
and J. L. Gonçalves
3
1
School of Electrical and Computer Engineering, University of Campinas, Av. Albert Einstein 400, Campinas, SP, Brazil
2
Center for Semiconductor Components, University of Campinas, R. João Pandiá Calógeras 90, Campinas, SP, Brazil
3
Center for Information Technology Renato Archer, Rod. D. Pedro I (SP – 65) Km 143, 6, Campinas, SP, Brazil
4
Superior School of Sciences of the Health, University of Amazonas, Av. Carvalho Leal, 1777, Manaus, AM, Brazil
Keywords: SiNx/SiO
2
, Silicon nitride thin film, ISFET, Chemical sensor, Biochemical sensor.
Abstract: In this work, nitrogen rich SiN
x
thin film was deposited on SiO
2
/p-Si (100) substrate by low pressure
chemical vapour deposition (LPCVD). The film was physically characterized using techniques such as
Fourier transform infrared spectroscopy (FTIR), atomic force microscopy (AFM) and ellipsometry. The
biocompatibility of such film was investigated by FTIR. Using a set of metal insulator semiconductor field
effect transistors (MISFETs) and ion sensitive field effect transistors (ISFETs) fabricated, electrical
characteristics and sensing properties were investigated. The biocompatibility of the SiN
x
film and the
electrical quality of the SiN
x
/SiO
2
/p-Si interface obtained suggests that SiN
x
/SiO
2
is an adequate insulator
on ISFET based chemical and biochemical sensors.
1 INTRODUCTION
LPCVD Si
3
N
4
films are used as the sensitive
material in miniaturized ISFET-based chemical and
biochemical sensors. Such devices have been used
for example for further surface modifications
allowing for antigen-antibody biosensor
applications. In vivo studies classify Si
3
N
4
as a
biocompatible material (Gustavsson et al., 2008).
The first ISFET was applied by Bergveld (Bergveld,
1970) to a biosensor for measuring ion concentration
in nerve tissues. The latest investigations related to
the ISFET-based biosensor are extended to
immunosensing (Schenck, 1978, Schöning and
Poghossian, 2002) and DNA hybridization sensing
(Souteyrand et al., 1997, Pouthas et al., 2004).
Ultrasensitive detection of biomolecules using
various types of one-dimensional nanostructures
such as carbon nanotubes (Villamizar et al., 2008),
graphenes (Cheng et al., 2010) and nanowires
(Knopfmacher et al., 2010) has attracted broad
research interest during the past decade due to their
high surface-to-volume ratio. An important
parameter, the slop of characteristic, is directly
related with sensitivity of the sensor. These aspects
have not been yet convincingly reported, for this
reason, this work reports the use of SiN
x
/SiO
2
stacked sensitive thin films that are biocompatible
and present high quality of the electrical interface,
increasing the sensitivity and making possible a
direct electrical detection of charged molecules.
2 EXPERIMENTS
2.1 SiN
x
/SiO
2
Stacked Sensitive Thin
Film Preparation
The (100)-orientated 1-10 Ω.cm p-type silicon
wafers were used as substrates. The 5 nm thick
thermally grown silicon oxide (SiO
2
) film was
prepared using a conventional furnace at 1000
o
C for
1 minute, in a high purity oxygen atmosphere. Then,
the SiN
x
layer, a sensing membrane, was deposited
by LPCVD in a SiCl
2
H
2
/NH
3
gas mixture
atmosphere at 740
o
C. The base and work pressure
of the chamber were 10 mTorr and 0.57 Torr,
respectively. The flow rate of SiCl
2
H
2
was fixed at
23 standard cubic centimeters per minute (sccm),
while the flow rate of NH
3
was 60 sccm. All
302
Souza J., Lima M., Doi I., Tatsch P., Diniz J. and Gonçalves J..
SINX/SIO2 STACKED SENSITIVE THIN FILM FOR ISFET-BASED CHEMICAL AND BIOCHEMICAL SENSORS - Preparation and Characterization of
the Stacked Thin Films and Sensors.
DOI: 10.5220/0003721603020306
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 302-306
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
deposition parameters are shown in Table 1.
Table 1: Parameters for the deposition of SiN
x
film.
SiCl
2
H
2
flow rate 23 sccm
NH
3
flow rate 60 sccm
Temperature of the substrate 740
o
C
Base pressure 10 mTorr
Work pressure 0.57 Torr
Deposition rate 27 Å/min
2.2 Immunoglobulin’s Self-assembled
Monolayer (SAM) Preparation
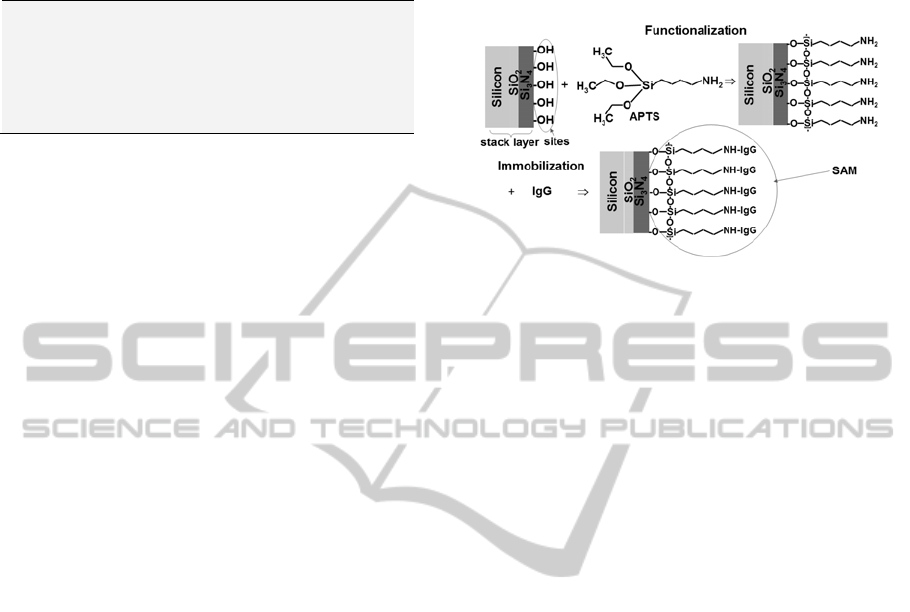
Figure 1 shows a schematic diagram of the method
used to prepare the substrates (functionalization).
The substrates were cleaned using a standard RCA
wet process. The 3-Aminopropyltriethoxysilane
(APTS) coating was carried out in a solution of 5%
APTS in toluene for 6 h, at 80
o
C. The non-adsorbed
APTS was removed by rinsing the substrate with
toluene and ethanol and dried with N
2
gas. The
APTS-modified substrates were baked in an oven at
110
o
C for 16 h. To form the immunoglobulin’s
SAM (Figure 1, immobilization), the substrates
coated with APTS were immersed into a solution of
ethylcarbodiimide (EDC), 2 mM, N-
hydroxysuccinimide (NHS), 5 mM, and
immunoglobulin - IgG, 1 μg/ml, at room
temperature for 2 h, and then washed with phosphate
buffered saline (PBS) of pH 7.2 and deionized water
(18 MΩ).
2.3 MISFET and ISFET Structures
Fabrication
For the MISFET and ISFET the SiN
x
/SiO
2
stacked
sensitive thin film were used as gate dielectric on p-
type (1-10 Ω.cm) Si (100) substrate. The substrates
were cleaned using a standard RCA wet process.
The critical steps in this fabrication procedure are as
follow:
1) Thermal gate oxide: 50 Å;
2) SiN
X
deposition by LPCVD: 300 Å;
3) SiN
X
dry etching and oxide wet etching of
the source and drain regions;
4) Al etching of gate electrode; and
5) Entire device coated with insulating layer to
eliminate ionic short circuits due to
exposure to solutions.
2.4 SiN
x
Layer Characterizations
The SiN
x
thin films used in these studies were
characterized by FTIR (infrared spectra with 32
scans and 4 cm
-1
resolution), AFM in contact image
mode (surface morphology), and ellipsometry (film
thickness and refractive index).
Figure 1: Schematic diagram of the preparation of the
substrates (functionalization) and formation of the
immunoglobulin’s SAM (immobilization).
2.5 IgG’s SAM Characterization
After immunoglobulin’s SAM preparation, Fourier
transform infrared spectra were performed. All
spectra are collected with 32 scans and 4 cm
-1
resolution.
2.6 Measurements Setup
In order to study electrical and sensing properties,
current-voltage (I
DS
-V
GS
) and current-time (I
DS
-
time) of MISFETs and ISFETs structures were
measured by a semiconductor parameter analyzer
Keithley 4200-SCS. The gate voltages were applied
to an aluminium metal gate of MISFETs and a gold
reference electrode for ISFETs.
3 RESULTS AND DISCUSSION
3.1 Physical Characteristics of SiN
x
Film
The FTIR spectrum of SiN
x
(Figure 2) exhibits a
clearly pronounced peak at 828 cm
-1
and smaller
peak at 467 cm
-1
, which are typical for the Si-N
bond in amorphous silicon nitride (Beshkov
et al.,
2003). The deposition rate is 27 Å/min and the films
have high refractive index η= 2.0 indicating that the
films are nitrogen rich. The AFM image (Figure 3)
shows the formation of very smooth and uniform
SiN
x
films, with average (Ra) and root mean square
(Rrms) roughness of 0.31 nm and 0.46 nm,
respectively.
SINX/SIO2 STACKED SENSITIVE THIN FILM FOR ISFET-BASED CHEMICAL AND BIOCHEMICAL SENSORS -
Preparation and Characterization of the Stacked Thin Films and Sensors
303
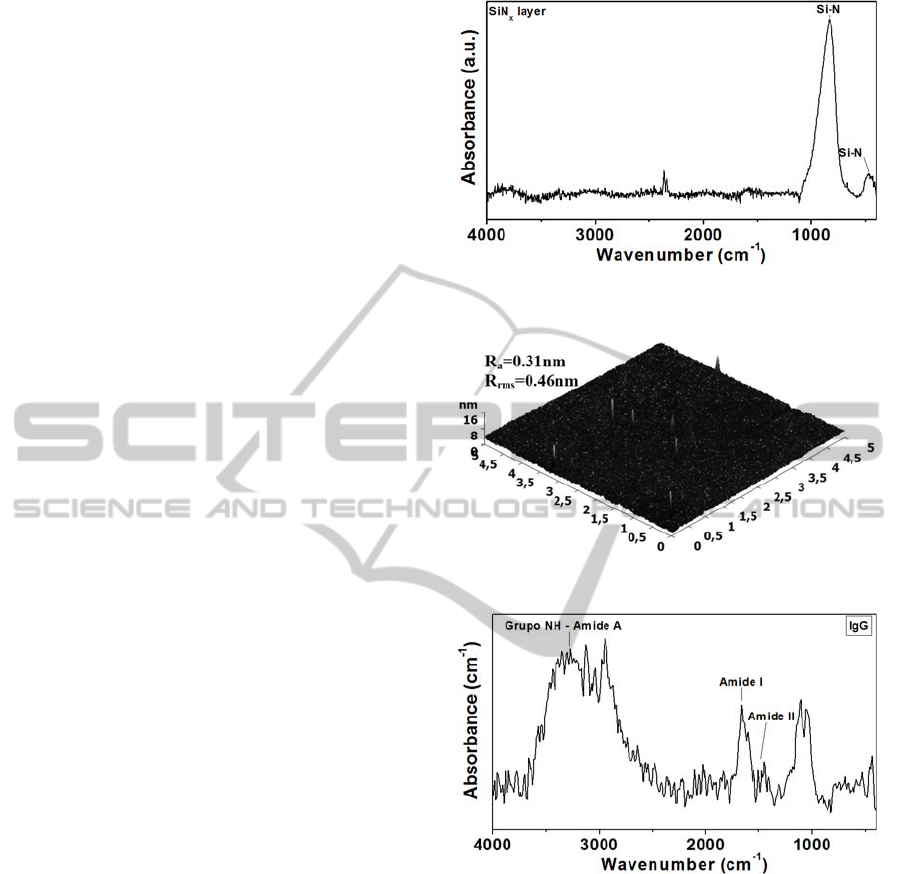
3.2 IgG’s SAM Characteristics
The characteristic vibrational peaks are mainly
dominated by the protein constituents of the IgG’s
SAM (Figure 4). A vibration band assignment is
done with the idea of the group frequencies of the
various analytes present in the SAM. The spectral
region 3600–3000 cm
-1
comprises of C-H, O-H, and
N-H stretching vibrations of the proteins. The
prominent absorption peak at 3300 cm
-1
is due to the
N-H stretching mode (amide A) of proteins. The
asymmetric and symmetric stretching C-H vibrations
of methyl and methylene group are found to be
present around 2930–2875 cm
-1
. The strong
absorption band at 1650 cm
-1
correspond to C=O
stretching vibrations (amide I) whereas the vibration
band at 1542 cm
-1
is attributed as amide II arising of
N-H bending vibrations strongly coupled with C-N
stretching of proteins. The absorption peaks in the
region 1400–1200 cm
-1
arise due to the C-H
deformation of methyl and methylene group of the
proteins. The spectral region 1250–925 cm
-1
is
predominantly occupied by C-O-C asymmetric and
symmetric vibrations of phospholipids of proteins
(Sankari et al., 2010).
3.3 Electrical Properties of Devices
MISFET – Electrical characteristics of MISFETs
including transconductance (G
m
), current-off (I
off
)
and subthreshold swing (S
t
) were calculated through
drain-source current versus gate-source voltage (I
DS
-
V
GS
) curves of the stack SiNx/SiO
2
gate MISFET
(Figure 5). The I
off
extracted at V
GS
=V
T
- 0.5 V was
2.17x10
-10
A. The calculated maximum
transconductance of the stack SiNx/SiO
2
gate
MISFET was 1.4 μS. S
t
is the slop of V
GS
versus log
I
DS
. The S
t
was obtained from the inverse of slope in
subthreshold region and is 147 mV/dec to the stack
SiNx/SiO
2
gate MISFET. These values are
acceptable for FET operation in an analog readout
circuit.
SENSING PROPERTIES – For pH sensitivity
calculation of the stack SiN
x
/SiO
2
gate ISFET, the
I
DS
vs. V
GS
, I
DS
vs. V
DS
and I
DS
vs. time curves in
saturation region were measured in standard pH
buffer solutions (pH 4, 7 and 10) at room
temperature. In Figure 6, the obvious linear shift of
I
DS
–V
GS
curves in different pH buffer solutions were
shown. The pH response and sensitivity was 50
mV/pH (I
DS
= 5 μA), so a quasi-Nernstian response.
Figure 2: FTIR spectrum of SiN
x
film deposited in
LPCVD reactor at 740
o
C with SiCl
2
H
2
and NH
3
.
Figure 3: Surface morphology of SiN
x
film on SiO
2
.
Figure 4: FTIR spectrum of immunoglobulin’s SAM.
The behaviour of current in function of time was
measured at constant drain-source and gate-source
voltage (V
DS
= V
GS
= 2V). As can be seen in Figure 7
and 8, the device showed an increase in current
when the pH value was increased. The pH sensibility
was 1.24 μA/pH. The linearity of the stack
SiN
x
/SiO
2
gate ISFET response is 97.4% in voltage
mode and 99.4% in current mode. Therefore, both
the pH responses are excellent in linearity. The
characteristic curves of the stack SiN
x
/SiO
2
gate
ISFET shows the normal FET operation and exhibit
the similar electrical characteristics as MISFETs.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
304
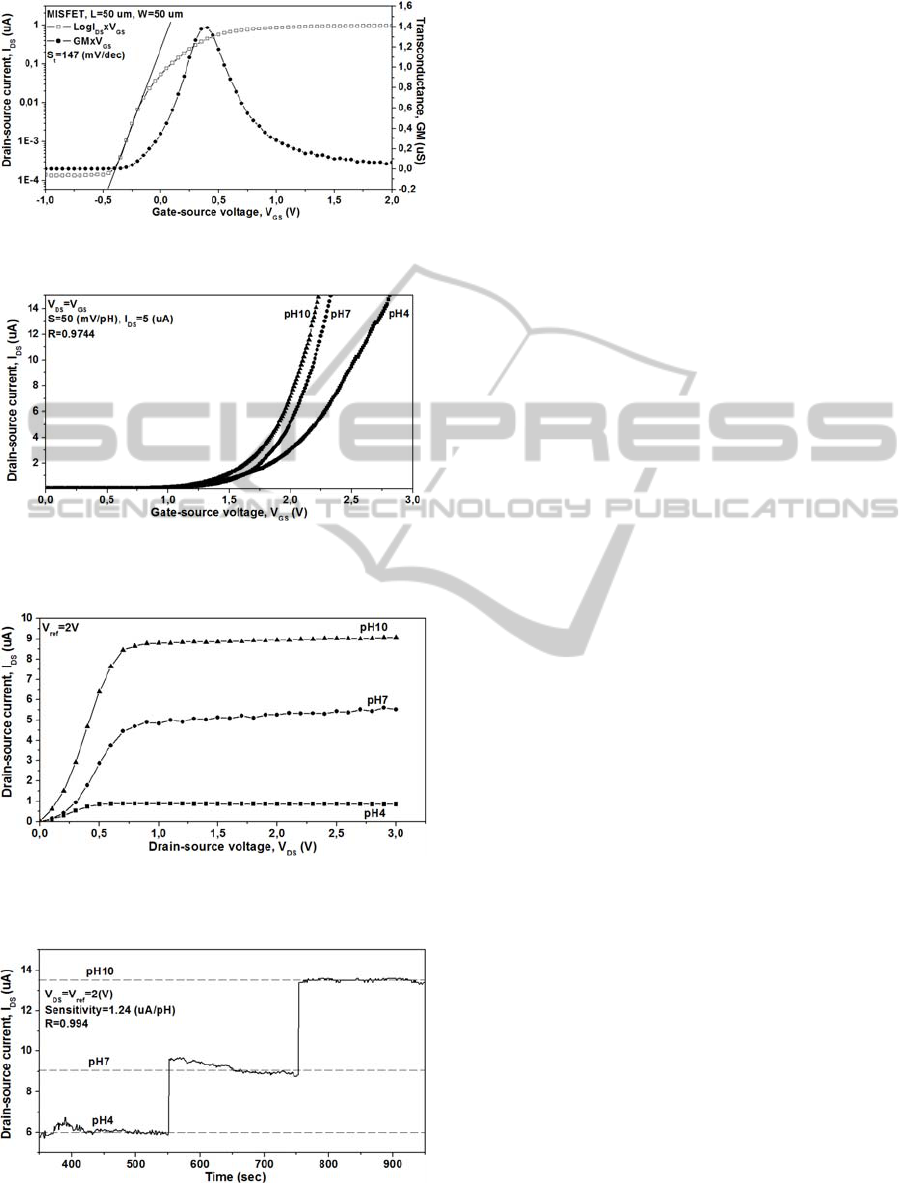
Figure 5: Log I
DS
vs. V
GS
and G
m
vs. V
GS
characteristics of
the stack SiN
x
/SiO
2
gate MISFET.
Figure 6: I
DS
vs. V
GS
curves of the stack SiN
x
/SiO
2
gate
ISFET measured at room temperature in standard pH
buffer solutions (pH 4, 7 and 10).
Figure 7: I
DS
vs. V
DS
curves of the stack SiN
x
/SiO
2
gate
ISFET measured at room temperature in standard pH
buffer solutions (pH 4, 7 and 10).
Figure 8: I
DS
vs. time curve in saturation region, measured
in standard pH buffer solutions (pH 4, 7 and 10) at room
temperature (V
DS
= V
GS
= 2V).
4 CONCLUSIONS
In this study, stacked sensing membrane with
LPCVD SiN
x
on SiO
2
was used for ISFET-based
chemical and biochemical sensors. The SiN
x
obtained was very smooth and nitrogen rich. By
fabricating and characterizing Immunoglobulin’s
self assembled monolayer we have demonstrated the
biocompatibility of the films. Electrical and sensing
properties were investigated by means of the stack
SiN
x
/SiO
2
gate MISFETs and ISFETs, respectively.
The results showed that the MISFET exhibits good
electrical characteristics. In regard to pH sensing
properties analysis, the stack SiN
x
/SiO
2
ISFET-
based sensor presented high performance with
almost Nerstian response (sensitivity of 50 mV/pH)
and high linearity of 97.4% in voltage mode and
99.4% in current mode. The obtained results
demonstrate therefore the feasibility of ISFET-based
sensors for the detection of charged molecules.
ACKNOWLEDGEMENTS
The authors would like to thanks the CCS staff for
technical assistance and the Brazilian agencies CNPq,
CAPES, FAPESP, and INCT-NAMITEC for the financial
support.
REFERENCES
Gustavsson, J., Altankov, G., Errachid, A., Samitier, J.,
Planell, J. A., Engel, E. (2008). Surface modifications
of silicon nitride for cellular biosensor applications. J.
Mater Sci: Mater Med, 19, 1839–1850.
Bergveld, P. (1970). Development of an Ion-Sensitive
Solid-State Device for Neurophysiological
Measurements. IEEE Transactions on bio-medical
engineering, 17, 70–71.
Schenck, J. F. (1978). In Cheung, P.W. (Ed.), Theory
Design and Biomedical Applications of Solid State
Chemical Sensors (pp. 165–173). CRC Press: Boca
Raton.
Schöning, M. J., Poghossian, A. (2002). Recent advances
in biologically sensitive field-effect transistors
(BioFETs). Analyst, 127, 1137–1151.
Souteyrand, E., Colarec, J. P., Martin, J. R., Wilson, C.,
Lawrence, I., Mikkelsen, S., Lawrence, M. F. (1997).
Direct Detection of the Hybridization of Synthetic
Homo-Oligomer DNA Sequences by Field Effect. J.
Phys. Chem. B, 101, 2980–2985.
Pouthas, F., Gentil, C., Côte, D., Bockelmann, U. (2004).
U. DNA detection on transistor arrays following
mutation-specific enzymatic amplification. Appl. Phys.
Lett., 84, 1594–1596.
SINX/SIO2 STACKED SENSITIVE THIN FILM FOR ISFET-BASED CHEMICAL AND BIOCHEMICAL SENSORS -
Preparation and Characterization of the Stacked Thin Films and Sensors
305

Villamizar, R. A., Maroto, A., Rius F. X., Inza, I.,
Figueras, M. J. (2008). Fast detection of Salmonella
Infantis with carbon nanotube field effect transistors.
Biosensors and Bioelectronics, 24, 279–283.
Cheng, Z., Li, Q., Li, Z., Zhou, Q., Fang, Y. (2010).
Suspended Graphene Sensors with Improved Signal
and Reduced Noise. Nano Lett., 10, 1864–1868.
Knopfmacher, O., Tarasov, A., Fu, W., Wipf, M., Niesen,
B., Calame, M., Schönenberger, C. (2010). Nernst
Limit in Dual-Gated Si-Nanowire FET Sensors. Nano
Lett., 10, 2268–2274.
Beshkov G., Lei S., Lazarova V., Nedev N., Georgiev S.
S. (2003). IR and Raman absorption spectroscopic
studies of APCVD, LPCVD and PECVD thin SiN
films. Vacuum, 69, 301–305.
Sankari G., Krishnamoorthy E., Jayakumaran S.,
Gunasekaran S., Priya V. V., Subramaniam S., Mohan
S. K. (2010). Analysis of serum immunoglobulins
using Fourier transform infrared spectral
measurements. Biology and Medicine, 2 (3), 42–48.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
306
