
PREPROCESSING IN MAGNETIC FIELD IMAGING DATA
Dania Di Pietro Paolo
BMDSys Production, Jena, Germany
Tobias Toennis
Medizinische Abteilung Asklepios Klinik St. Georg, Hamburg, Germany
Sergio Nicola Erne
BMDSys Production, Guenzburg, Germany
Keywords:
Magnetic field imaging, Magnetocardiography, Signal preprocessing.
Abstract:
Magnetic Field Imaging (MFI) is a new method of diagnosis of increasing importance in cardiology. MFI
records the magnetic fields (MF) of the electrical activity of the heart using many extremely sensitive sensors
and displays them afterwards in a clinically applicable manner. Due to the relatively low signal to noise ratio
(SNR) of the magnetic data, the recorded data are often averaged before analysis.
We describe a standardized preprocessing method to be used before averaging MFI data with low SNR. The
reported examples are data from 20 subjects out of a normal cohort examined at the Asklepios Klinik St.
Georg.
1 INTRODUCTION
Magnetic field imaging (MFI) is a new non-invasive
method that combines the recording of external mag-
netic field (MF) generated by the electrical activity of
the heart with its clinically applicable spatio-temporal
visualization. Cardiac MFs are very weak in compari-
son to the earth magnetic field and to electromagnetic
disturbances. To reduce the environment noise, mag-
netic acquisitions are normally carried out in magnet-
ically shielding rooms (MSRs). Unfortunately high
performances MSRs are very expensive and cannot
be easily integrated in the patient logistic of hospitals;
for this reason much effort has been made in order to
develop MFI systems usable in clinical environment.
To achieve this goal it has been necessary to redis-
tribute the task of noise reduction into the three main
components:
• shielding
• design of the sensor system
• data preprocessing
Here, the standardized preprocessing that is used for
collecting data routinely under clinical conditions is
presented.
2 MATERIALS
Twenty subjects (37± 14 years, 6 males, 14 females)
with no history of cardiac diseases have been selected
from a cohort of healthy controls. Written informed
consent has been obtained. The MFI recordings are
carried out at the Asklepios Hospital St. Georg in
Hamburg (Germany) using an Apollo CXS system
(BMDsys Production GmbH, Germany). Apollo CXS
is a 55-channels superconducting quantum interfer-
ence device (SQUID) gradiometer system arranged in
a hexagonal matrix, which covers an area of approxi-
mately 28 cm. The volunteers lie in a supine position
during the recording. The cryostat is placed at ap-
proximately 1 cm distance to the anterior chest wall
above the heart (Figure 1). The MFI sensor system
is operating inside a light magnetically shielded room
(Figure 2).
The data are sampled with a rate of 8200 samples
per second and stored at a sampling rate of 1025 Hz
(the bandwidth is set between 0.016 Hz and 256 Hz).
Recording duration is at least 180s.
463
Di Pietro Paolo D., Toennis T. and Nicola Erne S..
PREPROCESSING IN MAGNETIC FIELD IMAGING DATA.
DOI: 10.5220/0003160504630466
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2011), pages 463-466
ISBN: 978-989-8425-35-5
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
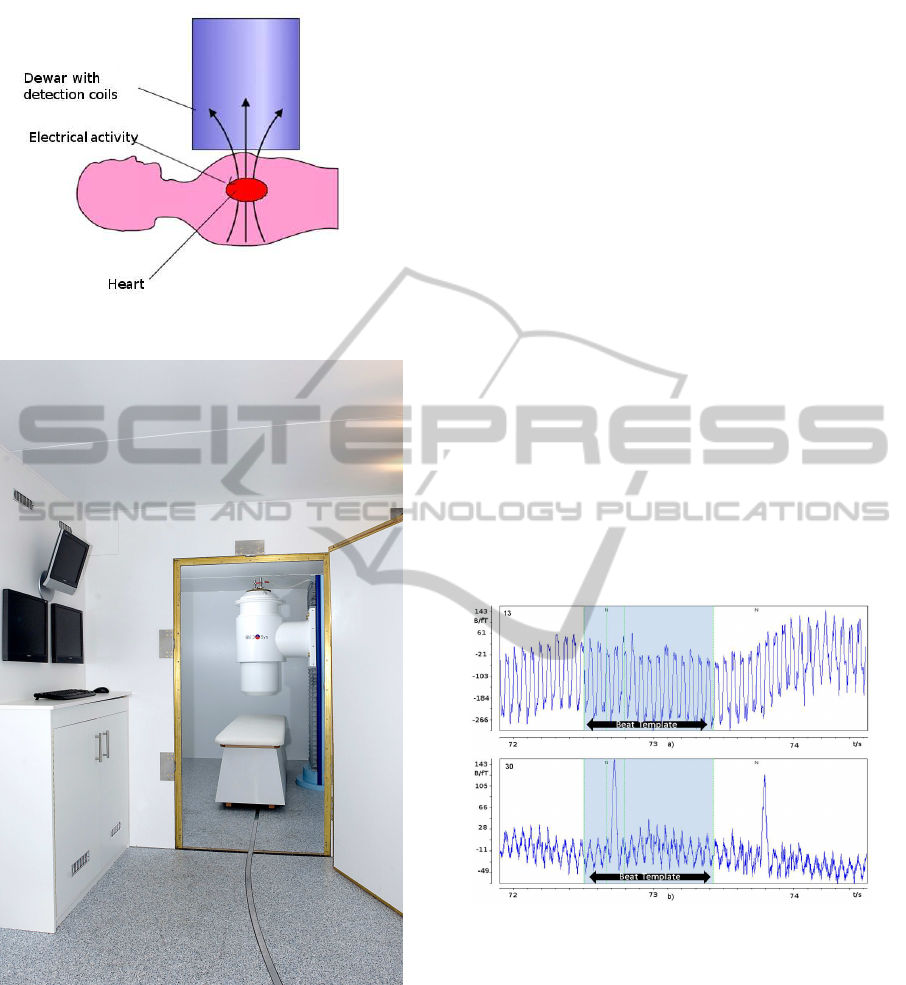
Figure 1: Model of MFI recording.
Figure 2: Biomagnetic system: view from the preparation
room into the acquisition room with patient bed and sensor
system.
3 METHODS
3.1 Real Time Preprocessing
The first step to be performed is disturbance subtrac-
tion. To compensate the noise generated by external
interferences, such disturbances are sensed by refer-
ences sensors and a suitable linear combination of
them is calculated for each channel. Then the result
of this operation is subtracted from the recording sen-
sors (Vrba, 1996). Successively, the signals are high
pass filtered using a RC type filter of the first order
with a time constant of 10 s. Before decimation and
low pass filtering, a proprietary adaptive comb filter
is used to eliminate the power line interference and its
harmonics. The comb filter introduces no phase shift.
Eventually, the data stream is decimated iteratively in
step of two to obtain the storing rate of 1025Hz. In
each decimation step an anti-aliasing filter with a rel-
ative cut-off frequency of 0.25 is used.
3.2 Segmentation
To perform averaging, the data stream has to be seg-
mented around each QRS complex. At the beginning
of the off-line analysis the operator iteratively selects
the suitable template parameters (Figure 3). Using the
template, the beats are selected according to the max-
imum coherence matching (MCM) algorithm. Then
the beats list is used as input in the categorized clus-
ter analysis (CCA).
Figure 3: Raw data in two channels: a) The upper panel
shows a channel with a transient vibration picked around
14-15 Hz, b) The lower panel shows a channel with very
high SNR. The beat with blue background is the beat used
for defining the template parameters.
3.3 Categorized Cluster Analysis and
Averaging
A modified version of CCA is used in order to find the
beats to be used in the averaging algorithm. The prob-
lem with the original version of CCA is that in case
the noise is homogeneously distributed over time, the
number of rejected beats can be very high since the
chosen beats are related to only one of the branches
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
464

Figure 4: Schematic illustration of a dendrogram which is
the graphical representation of the cluster analysis. Follow-
ing the arrows at each node, the largest group of beats with
the smallest similarity distance is found and thus the start-
ing point for function SNR. The horizontal axis represents
the observations (beats), the vertical axis gives the distance
(or dissimilarity measure).
(Figure 4). For this reason, in order to include more
beats to the average procedure, the distance matrix D,
used in the cluster analysis, is weighted using the av-
erage of the most similar beats in the branch taken
into consideration. At each step the closest beat is
added. In this way a larger number of beats for the
average can be reached when compared to the stan-
dard CCA. Using these beats the averaging procedure
is performed. For further information related to CCA,
please refer to (Di Pietro Paolo et al., 2005).
3.4 Transformation to a Standardized
Sensor Configuration
A localized source
~
j positioned 15 cm below the sen-
sor 0 (center of the sensor system) is used for con-
version based on multipole expansion (ME). Param-
eters till octupole, as proposed by Burghoff et all,
(Burghoff et al., 2000) are used in Cartesian space.
Using these terms, it is possible to reconstruct a 55-
channel averaged signal. A ME is a series expansion
that can be used to represent the field produced by
a source (in this case heart) in terms of expansion pa-
rameters which become small as the distance from the
source increases. Katila and Karp (Katila and Karp,
1983) proved the possibility to describe the heart sig-
nal by expansion of the magnetic multipoles up to the
octupole term. It is interesting to note that the mag-
netic dipolar term serves as a good approximation in
the early ventricular activation (Trontelj et al., 1991).
By adding more terms (quadrupoles and octupole) a
very accurate reconstruction of the sources can be ob-
tained. The magnetic multipole expansion as the fol-
lowing form:
B(~r) = −
µ
0
4π
Re
∞
∑
n=0
∞
∑
m=0
∇
1
r
n+1
P
m
n
(cosθ)e
−imζ
(1)
×
γ
mn
n+ 1
Z
∇
′
h
r
′
n
P
m
n
cosθ
′
e
imζ
′
i
x,~r
′
~
j
r
′
dv
′
Here are γ
mn
the coefficients so that (Katila and Karp,
1983):
γ
mn
= [2− δ(m)]
(n− m)!
(n+ m)!
with P
m
n
(cosθ) the associated Legendre functions of
first kind. The current density
~
j in the expression 1
is the total current density over the volume v
′
and the
expansion is valid for the region lying outside the sur-
face S
′
, i.e. r should exceed the largest value of r
′
.
4 RESULTS
The preprocessing is applied to the 20 acquisitions.
The performances of the preprocessing in terms of
noise reduction are measured using the Root Mean
Square (RMS) before and after the application of
CCA and transformation to a standard sensor con-
figuration. The SNR has been applied on the av-
eraged magnetic signals in the segment [T-end, P-
onset], since in this region the signal amplitude is usu-
ally very low. A summary of the results is shown in
table 1. The application of CCA and ME improved
the SNR of the averaged magnetic signals in almost
all cases up to 90%. Only in one case the preprocess-
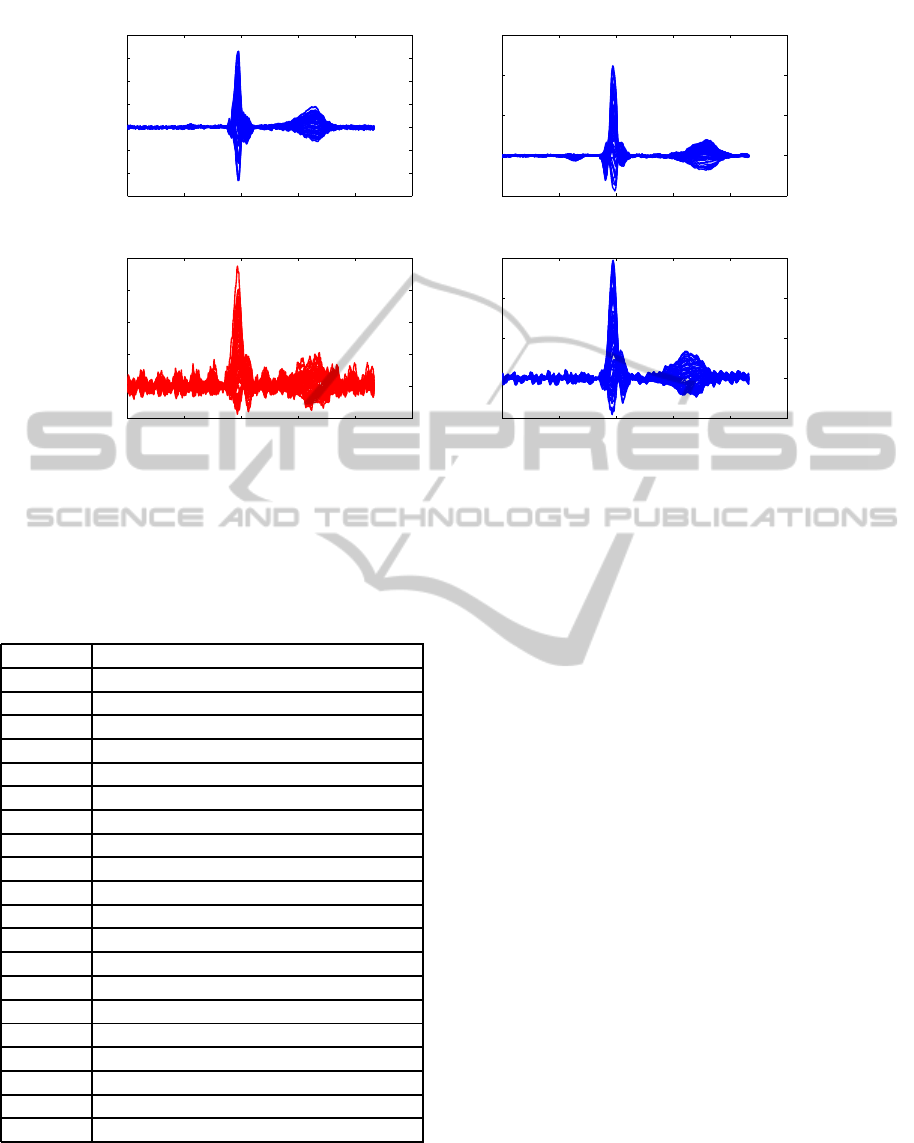
ing was not successful. Examples of averaged signals
are shown in Figure 5.
5 DISCUSSION AND
CONCLUSIONS
The preprocessing of MFI data outlined here is the
basis for all specific analysis that can be performed
with the MFI software of Apollo CXS (QRS frag-
mentation analysis, digital subtraction MFI for stress
induced ischemia detection etc). It has been shown
that combining a carefully designed low cost shielded
room (patent DE 10 2007 017 316 B4) with a gra-
diometric sensor system it is possible to obtain data
usable in clinical environment. Furthermore, the use
of a dedicated algorithm for the averaging procedure
PREPROCESSING IN MAGNETIC FIELD IMAGING DATA
465
0 200 400 600 800 t/ms
−6
−4
−2
0
2
4
6
8
x 10
4
a)
B/fT
0 200 400 600 800 t/ms
−5
0
5
10
15
x 10
4
b)
B/fT
0 200 400 600 800 t/ms
−5
0
5
10
15
20
x 10
4
c)
B/fT
0 200 400 600 800 t/ms
−5
0
5
10
15
x 10
4
d)
B/fT
Figure 5: Averaged magnetic signals using Apollo CXS: a), b) averaged magnetic signals after CCA and transformation to
standard sensor configuration (Subject 6 and 7, respectively); Subject 10 before (red) c) and after d) (blue) application of CCA
and ME.
Table 1: Noise reduction percentage in the 20 subjects after
applying CCA and standard sensor configuration transfor-
mation
Subjects Noise Reduction (%) after CCA + ME
1 33,76%
2 61,44%
3 65,05%
4 53,62%
5 -43,00%
6 74,52%
7 60,08%
8 35,49%
9 64,87%
10 68,29%
11 91,99%
12 62,83%
13 15,39%
14 56,98%
15 33,27%
16 14,14%
17 91,48%
18 16,73%
19 40,14%
20 91,28%
and the transformation to standard sensor configura-
tion make it possible to obtain data of quality com-
parable to those obtained in much more complicated
and expensive systems.
REFERENCES
Burghoff, M., Nenonen, J., Trahms, L., and Katila, T.
(2000). Conversion of magnetocardiographic record-
ings between two different multichannel squid de-
vices. Trans. Biol. Eng., 47(7):869–875.
Di Pietro Paolo, D., M¨uller, H.-P., and Erne, S. N. (2005).
A novel approach for the averaging of magnetocar-
diographically recorded heart beats. Phys Med Biol,
50(10):2415–2426.
Katila, T. and Karp, P. (1983). Magnetocardiography: Mor-
phology and multipole presentations. In Biomag-
netism: An interdisciplinary approach, pages 237–63.
S J. Williamson, G L. Romani, L Kaufman, I Modena.
Plenum Press, New York.
Trontelj, Z., Jazbinsek, V., Erne, S., and Trahms, L. (1991).
Multipole expansions in the representation of current
sources. Acta Oto-laryngologica, 111:88–93.
Vrba, J. (1996). SQUID gradiometers in real environments.
In Weinstock, H., editor, SQUID Sensors: Funda-
mentals, Fabrication and Application, pages 117–178.
Kluwer Academic Publishers.
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
466
