
POSITIONING AND ORIENTATION OF ADHERENT CELLS
IN A MICROFLUIDIC CHIP USING THE MICRO PATTERNING
OF A PARYLENE-C FILM
Claire Dalmay, Jun-Jung Lai, Laurent Griscom, Olivier Français, Bruno Le Pioufle
CNRS SATIE, IFR d’Alembert, ENS Cachan, Cachan, France
Frédéric Subra, Patrick Tauc
CNRS LBPA, IFR d’Alembert, ENS Cachan, Cachan, France
Joseph Lautru
IFR d’Alembert, ENS Cachan, Cachan, France
Keywords: Parylene-C patterning, Cell patterning, Cell orientation, Cell migration.
Abstract: A new method for the positioning and orientation of adherent cells on a culture substrate is presented. We
demonstrate the ability of a micro patterned parylene-C film deposited on a fused silica substrate to position,
isolate and/or orientate cells. Such features are crucial for the development of future biodevices for the
analysis and treatment of single-cell or organized cell tissues. In particular, our method is advantageous for
controlling the orientation of the cells within an organized tissue while being exposed to an electrical field.
The developed method does not require any chemical treatment of the cells or any additional surface
modification and is suitable for integration into a microfluidic system.
1 INTRODUCTION
This paper reports a novel method, based on
parylene-C patterning, for on-chip cell positioning.
This new approach finds various applications such
as i) the orientation of cells on biodevices ii) the
study of cell motility.
Classically, cell positioning can be achieved
thanks to different methods. Different works report
on the use of specific (as Fibronectin) or non-
specific (as poly-L-lysine) attachment biomolecules
(Ruiz, 2009 and Vogt, 2003). These biomolecules
are commonly patterned on the culture substrate by
microcontact printing process. Another solution
consists of using a high magnetic field (up to 10 T)
value during cell growth. In these conditions, cell
orientation is aligned in the same direction as
magnetic field (Umero, 2001). Nevertheless, the
efficiency of this kind of method for adherent cells
requires (i) the application of a strong magnetic field
and (ii) long exposure times (Hiroko, 2000).
Figure 1: Photograph of NIH-3T3 EWS/fli fibroblasts
cultured on a substrate of fused silica covered with two
repulsive lines of parylene-C.
In parallel, the use of parylene-C polymer in
biodevices has encountered an increasing interest
due to its biocompatibility, transparency, conformity
properties and long term stability (Shin, 2003 and
212
Dalmay C., Lai J., Griscom L., Français O., Le Pioufle B., Subra F., Tauc P. and Lautru J..
POSITIONING AND ORIENTATION OF ADHERENT CELLS IN A MICROFLUIDIC CHIP USING THE MICRO PATTERNING OF A PARYLENE-C FILM.
DOI: 10.5220/0003156702120215
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 212-215
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
Osaki, 2009). Some current cell-patterning
approaches use parylene-C as a stencil to achieve
patterns of cells (Wright, 2007 and Tan, 2009). In
these cases, the parylene-C film is peeled-off after
the cells have grown on the substrate in area
delimited by etched windows. This method is also
used to pattern proteins on a glass substrate, with
various high resolution shapes (Atsuta, 2007).
Nevertheless, these approaches are not directly
compatible with integration in a functional
microfluidic chip as (i) the peeling of the parylene
film inside a fluidic chamber or channel is not
permitted (ii) the adherent cells progressively spread
out from the initial patterns once the parylene stencil
is removed.
Consequently, alternative methods are required
to reach cell patterning techniques well adapted to
the use in biodevices including microfluidic systems.
We show in this paper that differences in surface
properties of parylene-C versus fused silica,
combined with the use of specific nanosized or
microsized patterns is capable of inducing precise
cell patterns, as well as the orientation of cells
towards specified directions. Our protocol does not
require the removal of the parylene-C film after the
cell culture, as the micro-patterned parylene-C film
functionalizes the biochip surface on which cells are
directly grown. The method benefits from several
parylene properties like biocompatility, biostability,
chemical inertness and hydrophobic nature.
Previous works have already demonstrated that
the nature of the substrate, as rigidity for example,
influences the cell movement (Lo, 2000). Here, we
show that the difference in cell adhesion affinity
between parylene-C and a fused-silica substrate
provoques cell migration towards fused silica
surfaces and induces their adhesion on the silica
surface (Figure 1). The paper highlights that the cells
are repulsed from small parylene structures, both in
the case of dots or micro-holes as long as the size of
the patterns is an order of magnitude lower than the
cell size.
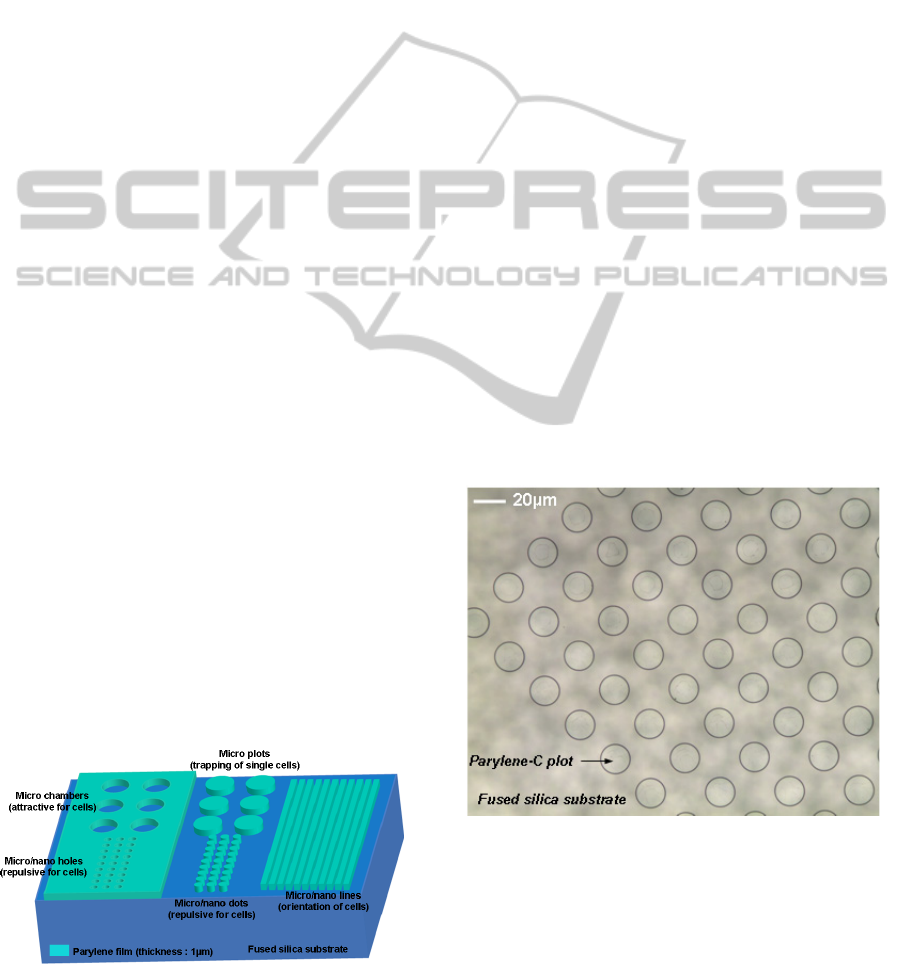
Figure 2: Schema of the patterned parylene film onto the
fused silica substrate (arbitrary scale).
Moreover, the patterning of different shapes -lines or
plots - within the parylene film (Figure 2), leads to a
preferential orientation of cells on the substrate
(typical result on figure 8).
2 EXPERIMENTAL SETUP
2.1 Fabrication Process
The chip is fabricated using classical
photolithography process for micropatterning the
parylene-C. In case of thin film Parylene-C layer
(1μm), we used Shipley S1818 photoresin as a
masking layer. So first, a uniform layer of parylene-
C, 1µm thick is deposited on a 2-inch diameter fused
silica substrate using a Specialties Coating Systems
Labcoater. The deposition is made by Chemical
Vapor Deposition (CVD) after a silanization step,
which ensures a good adhesion of the film. Patterns
defined by a standard photolithographic process
using Shipley S1818 positive photoresin and are
subsequently etched through the parylene-C layer by
oxygen plasma Reactive Ion Etching (Figure 3). For
thicker parylene layers (above 3μm), aluminum
layer (200nm) is used as interlayer mask since
S1818 is etched proportionally to parylene. To
finish, the biochip is sterilized under UV light to
ensure that no contamination appears during the cell
culture.
Figure 3: Photograph of a 1µm thick parylene-C film after
patterning.
2.2 Material and Methods
Biological tests have been performed using NIH-
3T3 EWS/fli fibroblasts which present a high
motility (Figure 4). These cells have been specially
POSITIONING AND ORIENTATION OF ADHERENT CELLS IN A MICROFLUIDIC CHIP USING THE MICRO
PATTERNING OF A PARYLENE-C FILM
213

chosen for their good properties of adherence and
migration.
First, cells are grown in standard culture wells
using Minimum Essential Medium supplemented
with 10% fetal bovine serum and 10% streptomycin
at 37°C in a humidified 5% CO
2
- 95% air incubator.
Figure 4: Photograph of a classical NIH-3T3 EWS/fli
fibroblast culture.
Then, cells are collected and counted in order to
be cultured within the biochip with the convenient
concentration.
The chip is firstly placed into a Petri dish and
immersed in the culture media. Then, approximately
200 000 cells are added into the media on the
surface of the chip. A few hours are necessary to
achieve cell adhesion. Cell behaviors such as
migration, adhesion and orientation are then
monitored during several days, using time-lapse
microscopy, for further analysis of the impact of the
micro patterned surface on their behavior.
3 BIOLOGICAL RESULTS
Biological experimentations led on these types of
micro patterns pointed out several phenomena,
which could be used advantageously for cell
positioning or orientation once integrated in
biodevices.
First, we show that it is possible to trap cells in
specific areas of the biochip as shown in Figure 5 (to
compare to the control experiment figure 4), thanks
to the migration and preferential adhesion of cells
towards fused silica surfaces versus parylene-C
areas.
Figure 5: 20µm Diameter dots made of parylene arrayed
with a 20µm step on a fused silica substrate. Photograph
of cell patterning after 82 hours in culture.
Plots arrayed with a distance comparable to the
cell diameter induce single cell patterning (which is
the case on Figure 5).
Secondly, we demonstrate that cells are strongly
repulsed from the areas covered with micro-dots
made of parylene arrayed on fused silica substrate
(figure 6.a.). In the same way, arrays of micro-holes
etched in the parylene film, down to the fused silica
substrate, induce the repulsion of cells (Figure 6.b).
These assumptions are verified as soon as patterns
characteristic sizes are equal or less than 5 μm.
Figure 6: Cells are repulsed from arrays of micro-dots or
micro-holes (a) 5µm diameter dots made of parylene,
arrayed with a 5µm step on a fused silica substrate. Cell
patterning after 82 hours in culture (b) 5µm diameter
micro-hole etched in the parylene film down to the fused
silica substrate, arrayed with a 5µm step. This network
(8x8 holes) is itself arrayed with a 75µm step. Phase
contrast image of cells patterning after 88 hours in culture.
Thirdly, we realized time-lapse imaging of cells
cultured on the micro patterned substrate for 15
hours. This experiment allowed us to observe the
migration of cells towards the fused silica and away
from the parylene surfaces (as shown in Figure 7).
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
214

Figure 7: Images of cell division and migration over a
span of 1h40min – cells are kept in sterile conditions
under 5% C0
2
at 37°C – Migrating cells are dark circled.
After 10 minutes dark circled cells divided. Then divided
cells started to send their pseudopods towards fused silica
areas where they finally migrate.
Finally, we show that the micro-patterning of
parylene induces the orientation of cells on the
substrate, as demonstrated on figure 8 where an
array of thin lines (typical width between 1 to 3μm,
spaced every 2μm) of parylene-C is performed on a
fused silica substrate. As shown on Figure 8, cells
orient themselves in parallel to the lines and grow
along the parylene lines (phase contrast image,
compared to a cell culture made on a non-patterned
substrate shown in Figure 4).
Figure 8: Phase contrast image of cells after 38 hours in
culture. On the left side the cells orient themselves along
2µm spaced network of 2µm width parylene lines. On the
right side of the images the cells cultured on a plain silica
surface display a random orientation.
4 CONCLUSIONS
These results demonstrate the high capability of
micro-patterned parylene film on fused silica
substrate to position and orientate adherent cells.
The developed technique can be easily adapted for
use inside a microfluidic system making it very
attractive in the biodevice field. It might be used
advantageously in cell biochips where a physical,
chemical, or electrical solicitation is applied to
cultured cells, as these activations may be sensitive
to the cell orientation (like electroporation chip for
instance). In addition, the possibility to isolate cells
may be very promising in the development of
biodevices for single-cell analysis.
REFERENCES
Atsuta, K., Suzuki, H., Takeuchi, S., 2007. A parylene lift-
off process with microfluidic channels for selective
protein patterning. J. Micromech. Microeng., 17, 496.
Hiroko, K., Masakazu, I., Shogo, U., 2000. Orientation of
adherent cells under magnetic fields and medical
applications. Papers of technical meeting on
magnetics, IEE Japan, 86, 19.
Lo, C.M., Wang, H.B., Dembo, M., Wang, Y.L., 2000.
Cell movement is guided by the rigidity of the
substrate. Biophysical Journal, 79, 144.
Osaki, T., Suzuki, H., Pioufle, B. L., and Takeuchi, S.
(2009). Multichannel simultaneous measurements of
single-molecule translocation in ˂-hemolysin
nanopore array. Analytical Chemistry 81.
Ruiz, A., et al., 2009. Single stem cell positioning on
polylysine and fibronectin microarrays. Micro and
nanosystems, 1, 50.
Shin, Y. S., et al., 2003. PDMS-based micro PCR chip
with Parylene coating. J. Micromech. Microeng., 13,
768.
Tan, C. P., Ri Seo, B., Brooks, D. J., Chandler, E. M.,
Craigheadb, H. G., Fischbach, C., 2009. Parylene peel-
off arrays to probe the role of cell–cell interactions in
tumour angiogenesis. Integr. Biol., 1, 587.
Umeno, A., Kotani, H., Iwasaka, M., Ueno, S., 2001.
Quantification of adherent cell orientation and
morphology under strong magnetic fields. IEEE
Transactions on Magnetics, 37(4), 2909.
Vogt, A. K., Lauer, L., Knoll, W., Offenhäusser, A., 2003.
Micropatterned substrates for the growth of functional
neuronal networks of defined geometry.
Biotechnology progress, 19(5), 1562.
Wright, D., Rajalingam, B., Selvarasah, S., Dokmecid, M.
R., Khademhosseini, A., 2007. Generation of static
and dynamic patterned co-cultures using
microfabricated parylene-C stencils. Lab Chip, 7,
1272.
POSITIONING AND ORIENTATION OF ADHERENT CELLS IN A MICROFLUIDIC CHIP USING THE MICRO
PATTERNING OF A PARYLENE-C FILM
215
