
AN RFID-BASED DRUG MANAGEMENT SYSTEM
A case in Medical Organization
S. L. Ting, S. K. Kwok, Albert H. C. Tsang and W. B. Lee
Department of Industrial and Systems Engineering, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China
Keywords: Case Study, Radio Frequency Identification (RFID), Drug Management System.
Abstract: Drug safety has attracted considerable public concern and press attention in recent years. Many precautions
have been suggested to ensure the accuracy of drug distribution to patients but the results are not yet fully
satisfactory. Enhancement of drug management is undoubtedly a trend to ensure no medication error happen
in the healthcare industry, but there is still plenty of room for improvement in this situation. Drug
Management System (DMS) is proposed as a platform for the hospital and clinics to explore the drug safety
and patient safety. This paper will present the details about DMS for assisting the medical workers in drug
replenishing and dispensing process. Also, problems existing in drug safety and benefits bring from DMS
are presented.
1 INTRODUCTION
Medicine is regarded as a tool to heal people with
sickness, but it is a weapon that can kill people. If
humans take in wrong medicine, they will have side
effects or eventually die. To avoid such mishaps,
effective drug management is very important to
healthcare industry.
Drug management involves two processes,
namely Drug Quality and Replenishing Process
(DQRP), and Drug Dispensing Process (DDP). To
enhance safety in these two processes, improving
human inspection is one of the common methods
applied in medical organizations. Despite
enhancements of inspection and quality of standards,
numerous poor drug management incidents have
been reported frequently in recent years. This
situation is particularly much more serious in clinics
compared with hospitals. This can be attributed to
the lack of monitoring system in drug management
adopted at clinics. As a result, medication errors
happened inevitably. In 2008, the Department of
Health reported that misidentifying medicines is a
common mistake (Department of Health, 2008).
Four clinics in Hong Kong have 442 medication
error events in two months with an average of seven
events a day. 260 medication error events were
related to wrong packaging (Tam et al., 2008). Some
medication errors brought serious impact to humans.
For example, in 2006, the clinic of a private general
practitioner in Hong Knog mistakenly mixed up the
syrup medicine for treating running nose with
Isopropyl alcohol in which it involves poison that is
normally used for wood processing. Another
example happened in last year: wrong drugs in packs
of medicine were found by mixing up diabetes
tablets with drugs for controlling high blood
pressure. About 60 patients were affected and they
were nearly killed because of drug allergy.
These medication error incidents have a common
characteristic – all the mistakes occurred and were
detected in the drug management processes, i.e.
DQRP and DDP. These processes involve the
knowledge of pharmacy. Drugs with similar shapes
and colors may have very different properties. For
example, Gasteel and Isodil, shown in Figure 1, have
similar shapes and colors but they are different on
the back side. The current practice of drug
identification mainly relies on visual inspection
performed by humans to determine the drugs. Unlike
the case in hospitals, clinics usually do not have
resident pharmacists. Instead, nurses usually play an
important role in the drug management processes.
With lesser medicinal knowledge and experience
compared with pharmacists, nurses are easier to
make mistakes in the drug management processes.
In short, if such processes contain errors, adverse
drug incidents will continue to happen even though
correct medications have been prescribed to patients.
In response to the above mentioned issue, a new
99
L. Ting S., K. Kwok S., H. C. Tsang A. and B. Lee W. (2010).
AN RFID-BASED DRUG MANAGEMENT SYSTEM - A case in Medical Organization.
In Proceedings of the Multi-Conference on Innovative Developments in ICT, pages 99-107
DOI: 10.5220/0003037700990107
Copyright
c
SciTePress

Drug Management System (DMS) is designed to
deal with the problems of medication errors. This
needs to modify the clinic operation by
implementing an RFID-based solution (Huang and
Ku, 2009; Ting et al., 2009; Fanberg, 2004). With
the automatic identification capability enabled by
RFID technology, the processes of drug
identification, drug distribution and drug processing
will be greatly improved. Moreover, the proposed
system can even detect whether the drugs are put in
the correct place or container, check the
compatibility of drugs for adverse interaction, and
deliver real time expiry date alert automatically.
In order to study the feasibility of our proposed
DMS, a Hong Kong medical organization is chosen
as case study. It specializes in providing health care
for ambulatory patients treated by several general
practitioners and medical professionals. Same as
many clinics around Hong Kong, its drug
management is a major challenge in its daily
operation. It would like to seek an effective and
accurate method to prevent medication errors.
Figure 1: Gasteel and Isodil – drugs that look alike at one
side.
2 MOTIVATION
AND OBJECTIVES
Four critical issues and challenges encountered in
current drug management practices are attempted to
address in this project:
Mixing Up of Drugs
In typical medical organizations, doctors and
nurses have to differentiate the drugs
frequently. It is because they need to replenish
the drugs when they are below the safety stock
level and dispensing drugs to patient when
electronic report is received. However,
concerning the shapes and colours of drugs are
similar (i.e. an example is shown in Figure 1);
it increases the challenges to distinguish the
drugs for human. Most of the errors occurred
because one drug is mixed up with another
drug. With the eyes and perception, the
mistakes of mixing up in the drugs
management cannot be tackled easily.
Therefore, it is necessary to apply technology
to facilitate the medical staff in drug
identification.
No Checking Expiry Date
As shown in Figure 2, the expiry date of drugs
is often printed on the original package of
drugs. After the packages of the drugs are
opened, the drugs of expiry date cannot be
tracked easily. Syrup will be found easily if the
drugs are deteriorated due to expired date. This
is because there are turbidities in syrup.
However, tablets will not have any appearance
changes after expiry date. Therefore, there is no
way to enhance the expiry date management
for the drugs.
Figure 2: Original Package of Drug with Expiry Date
Shown.
Lack of Drug-Drug Interaction
Some drugs cannot be prescribed to patients at
the same time as they will interact with each
other. In addition to the clinical knowledge and
experiences of physicians, a proper prescription
is also relied on the physicians’ understanding
of medicine properties, functions and
ingredients. With more and more new drugs are
available in the marketplace, medicine relation
becomes more complex. In this sense, doctors
may find difficult to recognize all these
relations and thus drug-drug interaction may
easily occur. In order to confront drugs adverse
interaction, technology can be used to facilitate
the physicians.
Based on the problem statements, the three major
objectives are:
INNOV 2010 - International Multi-Conference on Innovative Developments in ICT
100
To develop an effective and trusted drug
processing method to avoid the drugs mixing
up problems;
To provide a dynamic and evaluation platform
to assist the medical workers to check the
expiry date and drugs interaction of drugs; and
To facilitate collaborative and interactive drugs
replenishing and dispensing processes between
medical workers and patient.
3 ARCHITECTURAL
FRAMEWORK, STANDARDS
AND FUNCTIONS
OF THE RFID-BASED DRUG
MANAGEMENT SYSTEM
(DMS)
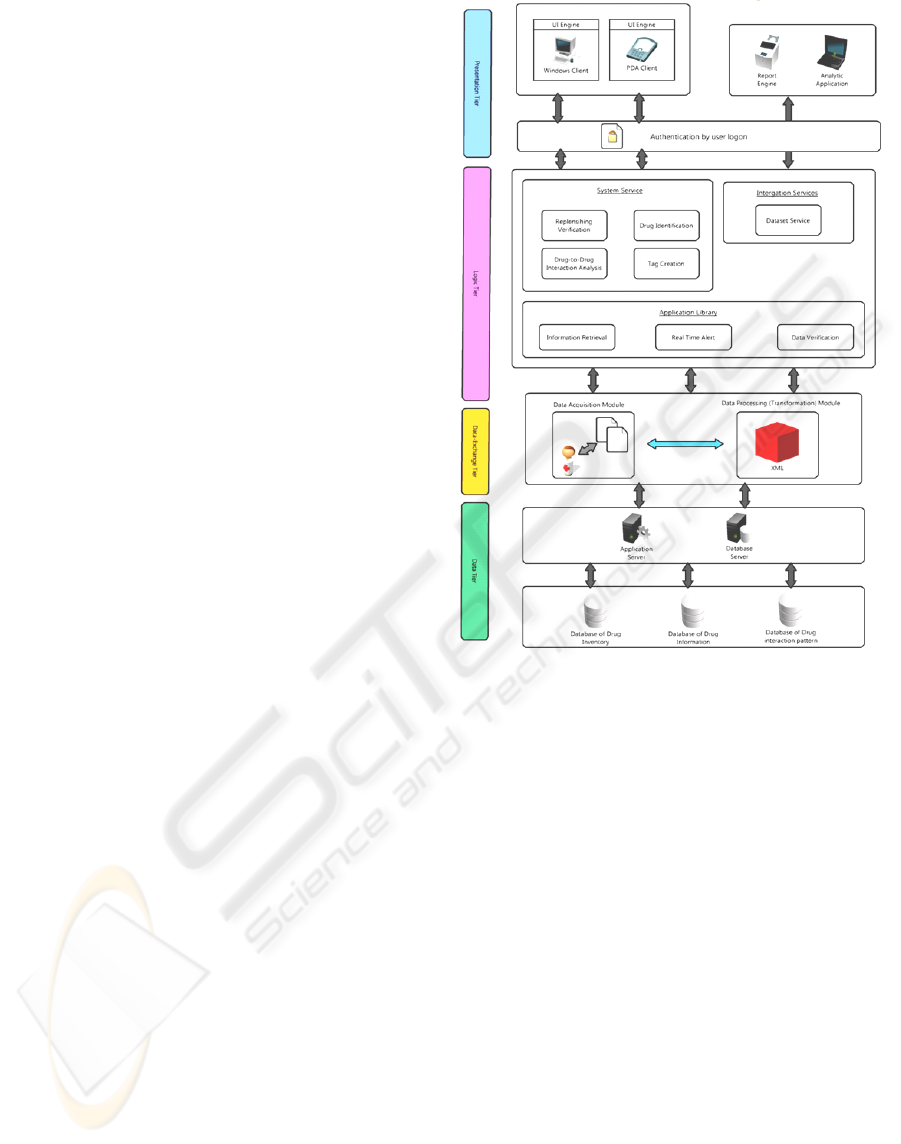
Figure 3 shows the system architecture of DMS.
Simply stated, the system is divided into 4-tiers
namely: Presentation Tier, Logic Tier, Data
Exchange Tier and Data Tier. The Presentation Tier
is used to deliver the response to the users; while the
Logic Tier processes and fuses the information
scanned by the RFID reader; then the collected raw
data is transforming into standard information by the
Data Exchange Tier and the Data Tier stores the
processed information (RFID tag information) for
further analysis.
3.1 Details of the System Architecture
The Presentation Tier represents the communication
media that enable users to receive essential
information in appropriate form, and to acquire
information based on the various authorities.
Generally, it includes input or output devices such as
UI Engine (like Personal Computers (PC) and
Personal Digital Assistants (PDA)) and Report
Engine (like Printer). Through a secure
authentication by user logon with Logic Tier, users
can enquire the system and obtain the information
like drug information, expiry date, and so on.
The Logic Tier is the brain of the whole system
since most of the work is performed here. It consists
of three major applications: Information Retrieval,
Real Time Alert and Data Verification. These
applications are useful in recording and retrieving
data in an intelligent and logical way. So that the
system can send out alert for enquiries after any
successful match between Logic Tier and Data Tier.
Moreover, these applications support difference
Figure 3: System Architecture of Drug Management
System (DMS).
services and can be classified into two categories:
System Service and Integration Service. The details
of the services are demonstrated as follow:
System Service
The System Service consists of 4 modules
which are Replenishing Verification, Drug
Identification, Drug-to-Drug Interaction
Analysis and Tag Creation. The Replenishing
Verification module responds for ensuring the
correctness between the two replenished
package and container; while the Drug
Identification module responds for retrieving
all the information of particular drug from drug
database; and the Drug-to-Drug Interaction
Analysis module responds for checking the
interaction; and finally, the Tag Creation
module responds for creating the tag once
drugs are received from manufacturers.
Integration Service
The Integration Service is mainly responsible
for report generation. That means useful
information can be extracted from suitable
database(s) by the Dataset Service module and
AN RFID-BASED DRUG MANAGEMENT SYSTEM - A CASE IN MEDICAL ORGANIZATION
101
they will be presented by the Report Engine in
the Presentation Tier.
After the information processed by the Logic
Tier, it will then be transferred to the Data Exchange
Tier which is responsible for collecting raw data and
transforming them into standard information. The
Data Exchange Tier is constituted of 2 modules:
Data Acquisition Module and Data Processing
(Transforming) Module. For Data Acquisition
Module, it acquires raw data to the system. Each
RFID tag, on the package of drugs, containing tag
ID and drug information will be used to represent
each category of drug. When we use RFID reader to
detect them and once the reader received the
returned signal, the tag can be read. For Data
Processing Module, those collected data will be
transformed and normalize into a common format –
eXtensible Markup Language (XML). XML is a
common information format which can be used on
the Internet, Intranets and elsewhere. Upon the
completion of transformation process, the standard
information will be transferred to the Data Tier to
store the information.
The Data Tier consists of several databases
include Drug Inventory, Drug Information and Drug
Interaction Pattern that contain all the information of
the system. When a raw data is captured, it will be
first under processing in Logic Tier. Then it will be
converted to meaningful information and pass
through the Application Server or the Database
Server. Finally, the information will be stored in a
suitable database in Data Tier. When any enquiry is
made, the information will be selected from the
related database in Data Tier. The Data Tier is acting
as an information repository of the system.
3.2 RFID Technology Standards
In normal practice of medical organizations, there
are mainly eight types of medicine, including
capsules, tab, syrup, power, lozenges, gel, cream and
drops or lotion.
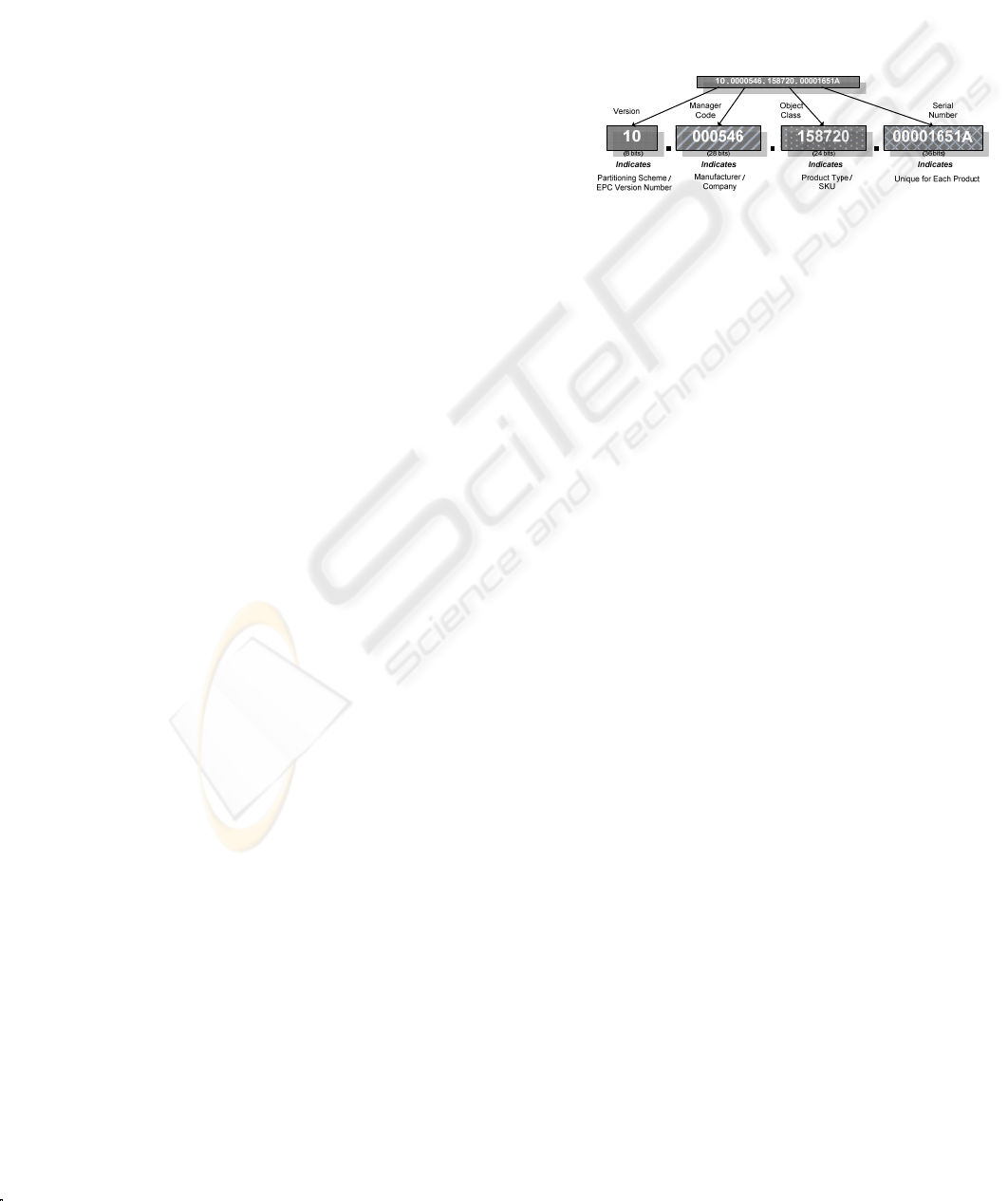
To standardize the numbering schema in the
DMS, Electronic Product Code (EPC) is adopted
(Kwok et al., 2008). EPC is a global unique serial
number that identifies products in item level
(EPCglobal Inc., 2005). As shown in Figure 4, EPC
consists of four components. Generally, it enables
users to store 96-bit data, which categories into
version, manager code, object class and serial
number. In order to store detail information of
medicine and enable medical staff to identify drugs
in item level, some critical information will be given
specific number as differentiation. The schema is
described as follow:
Version Section
It stores a 2-digit figure which can indicate
types of medicine from 01-08 (i.e. the typical
eight categories).
Manager Code and Object Class
They store 7-digit and 6 digit code
respectively. They record the other relevant
information like types of package and
manufacturing company for identification.
Serial Number
It stores a 9-digit code that represents the
assigned unique number for item identification.
Figure 4: Format of EPC Number (96-bit version).
There are various package combinations of
different types of medicines such as boxes or bottles.
If the exact volume can be identified, large packages
of drugs can be stored systematically. By assigning
an EPC numbering system in the clinic, exact
package or bottle of drugs can be easily identified or
found. Once the RFID reader detects an RFID tags,
the DMS can efficiently show related information
such as drugs’ name and quantity to the medical
staff.
3.3 System Functions
The proposed system performs 5 functions: Tag
Creation Function, Drug Identification Function,
Replenishing Verification Function, Expiry Date
Alerting Function and Drug-to-drug Interaction
Checking Function, they are described as Table 1.
4 CASE STUDY: APPLICATION
OF DMS IN A HONG KONG
MEDICAL ORGANIZATION
In order to study the feasibility of our proposed Drug
Management System, a Hong Kong medical
organization is chosen as case study. Traditional
workflow analysis is discussed first to illustrate the
problems encountered in drug management in the
organization, and hence the second part
demonstrates how the proposed methodology can
enhance the current situation and challenges.
INNOV 2010 - International Multi-Conference on Innovative Developments in ICT
102
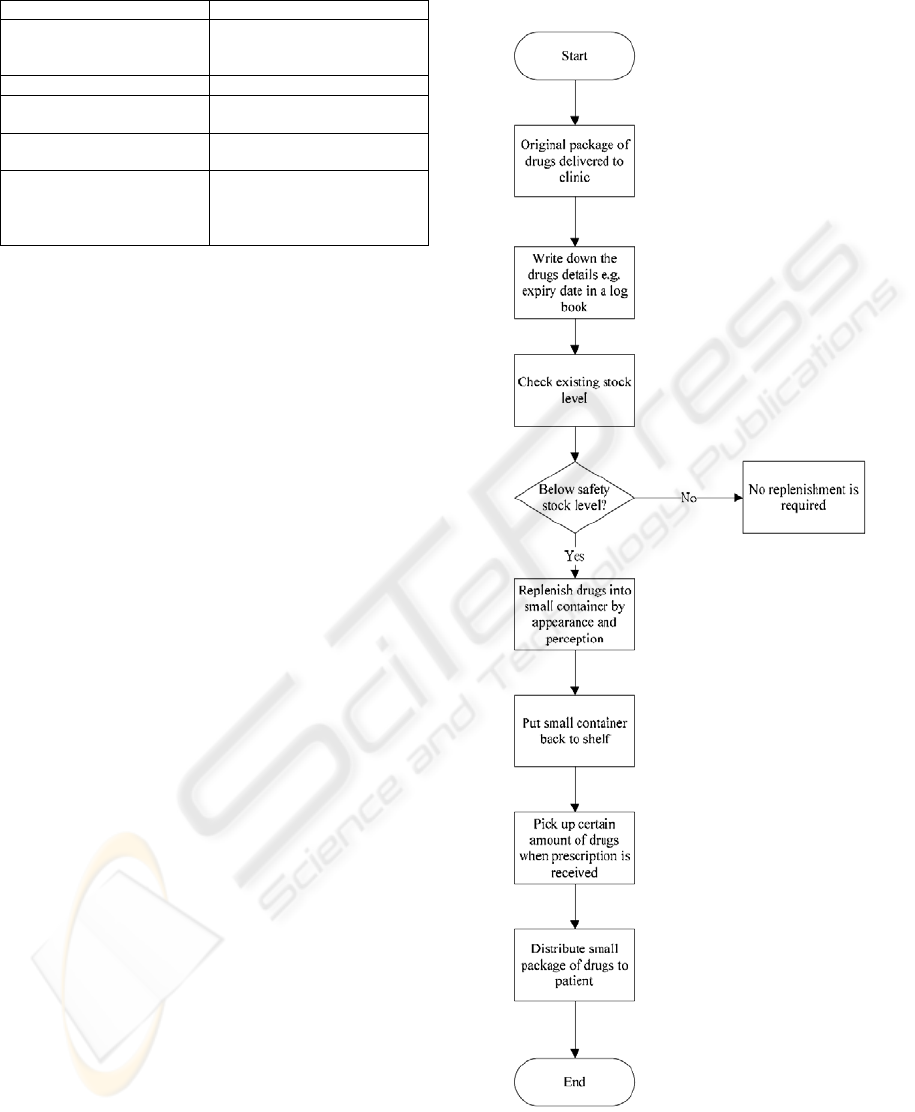
Table 1: System Functions of RFID-based DMS.
Function Description
Tag Creation To create the tags and input
relevant
drugs information
Drug Identification To review the drug details
Replenishing Verification To confirm the drugs being
replenished correctly
Expiry Date Alerting To determines whether the
drug is expired or not
Drug-to-drug Interaction
Checking
To determine whether the
medicines dispensed to
the patient have interaction or
not
4.1 Traditional Workflow Analysis
Figure 5 shows the existing working process of drug
management. Generally, drug management can be
divided into two processes, Drug Quality and
Replenishment Process (DQRP) and Drug
Dispensing Process (DDP).
When several large packages of drugs have been
first delivered to the clinic, medical workers (i.e.
usually nurses may take this role) may record the
relevant drugs information such as expiry date and
quantity in a logbook. In usual practice, large
packages of drugs are replenished into small
containers for stocking. After checking the existing
stock level of drugs, if the existing stock level is
lower than the safety stock level, large packages of
drugs are refilled into small containers. While
refilling drugs, nurses are required to match drugs
and small containers largely based on drugs’
appearance and their experiences. In a large extent,
human errors are made since there is not much
difference between drugs’ appearance and medical
staff may mistake small container and transfer pills
to an incorrect small container. Moreover, some
relevant information like expiry date of drugs needs
large human involvement to check from the
logbook. With the demanding services of clinic, it is
time consuming to realize the date first before any
replenishment is conducted. Once the refilling
process completed, the containers are stored back to
the shelf.
Another process is about drug dispensing which
regulates the procedure of dispensing drugs to
patients. Figure 6 shows the workflow of DDP. In
existing clinic operation, an Electronic Medical
Record (EMR) system is used to notice staff that
specific kinds of medicines should be distributed to
an exact patient (McInnes et al., 2006). After
receiving the record, nurses may pick up particular
small containers of drugs from the shelf and
distribute certain amount of drugs into small
package and finally distribute to patient.
Figure 5: Existing Workflow of DQRP.
AN RFID-BASED DRUG MANAGEMENT SYSTEM - A CASE IN MEDICAL ORGANIZATION
103
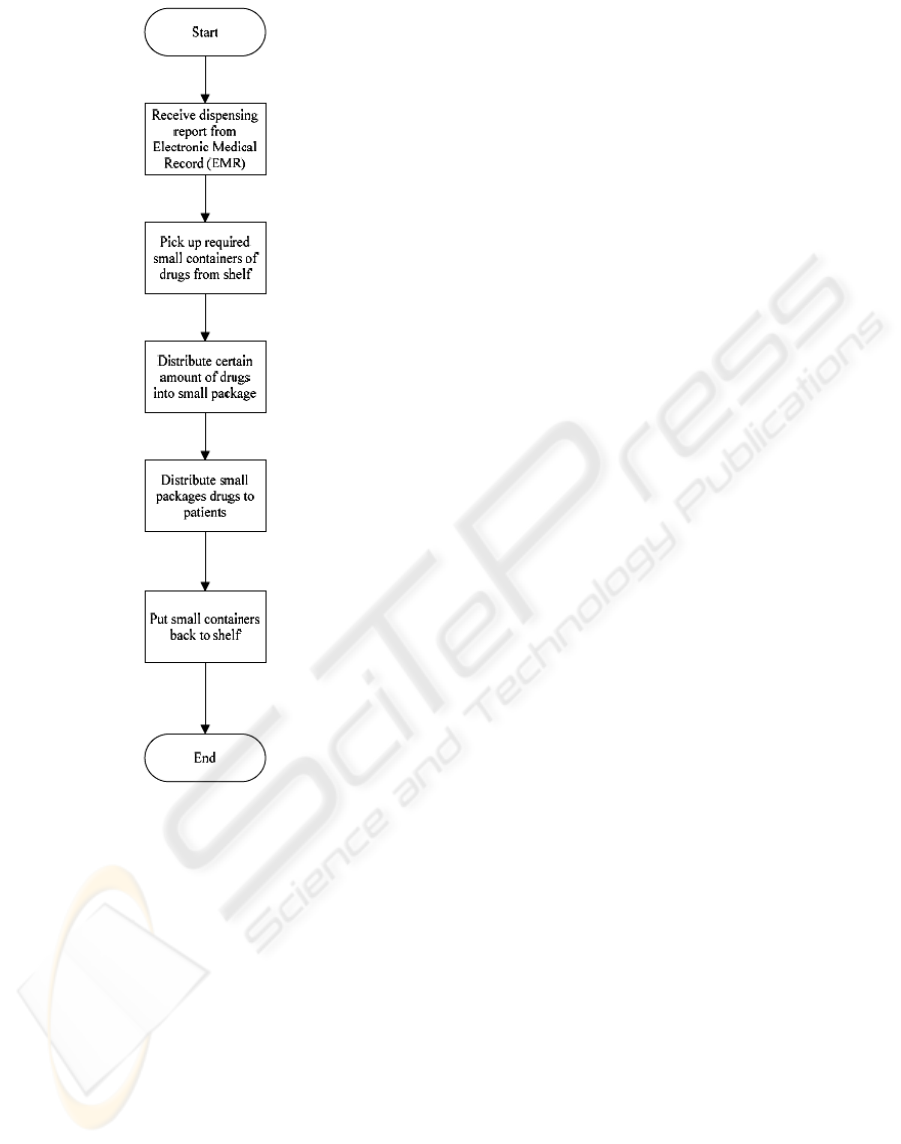
Figure 6: Existing Workflow of DDP.
4.2 Proposed Methodology
In order to tackle the problems that involved in the
DQRP, a DMS is introduced to the company. It is an
RFID-enabled system to manage the flow of drugs
throughout the chain. As large packages of drugs
have been delivered to the clinic, each package of
drugs is given a RFID tag with relevant information
stored in EPC standard. Since drugs are split into
different categories, the categories indicator is stored
as a part of identity according to EPC standard. By
then, after creating a unique RFID identity to each
package of drugs, drugs are refilled into small
containers if the existing stock level is lower than
the safety stock level. In order to ensure a correct
small container is picked, nurses are required to
place both large package of drug and small container
on an RFID reader for authentication. This is an
important step to make sure the right medicine will
be replenished into the right small bottle. Prior to
DMS, human errors are happened frequently since
the authentication process is highly depends on
worker’s perception and identification of drugs’
appearance.
If package of drugs can match with a small
container, nurses can start to replenish medicine into
small bottles. After refilling all medicines, small
containers are placed back to shelf.
For the proposed working procedure of DDP,
after receiving drugs dispensing report from EMR
system, nurses pick the right small containers from
shelf and place onto an RFID reader to ensure the
right bottles are selected. Meanwhile, expiry date
can be double examined and to prevent from
distributing expire medicine to patients. If drugs are
expired, they should be disposed immediately.
Moreover, the DMS enables users to detect whether
there is drugs interaction. If drugs are not interacted,
they can be distributed to patients.
4.3 System Functions
The proposed system performs 5 functions: Tag
Creation Function, Drug Identification Function,
Replenishing Verification Function, Expiry Date
Alerting Function and Drug-to-drug Interaction
Checking Function, they are described as follow:
Tag Creation Function
As shown in Figure 7, this function is in charge
of creating the tags and input relevant drugs
information once drugs received from
manufacturers.
Drug Identification Function
As shown in Figure 8, this function retrieves all
relevant information of particular drug from
databases so that the medical workers can
review the drug details and make sure the
replenished and dispensed drugs are correct.
Replenishing Verification Function
This function deals with confirming relevant
drugs mainly. It examines the tags on two
packages of drugs first, and then come to use
replenishing verification function. If the two
RFID tags are matched, a Green Tick will be
shown (Figure 9a); otherwise, a Red Cross will
be shown (Figure 9b). After the steps
completed, the medical workers are admitted to
transfer the drugs from one package to the
other.
INNOV 2010 - International Multi-Conference on Innovative Developments in ICT
104
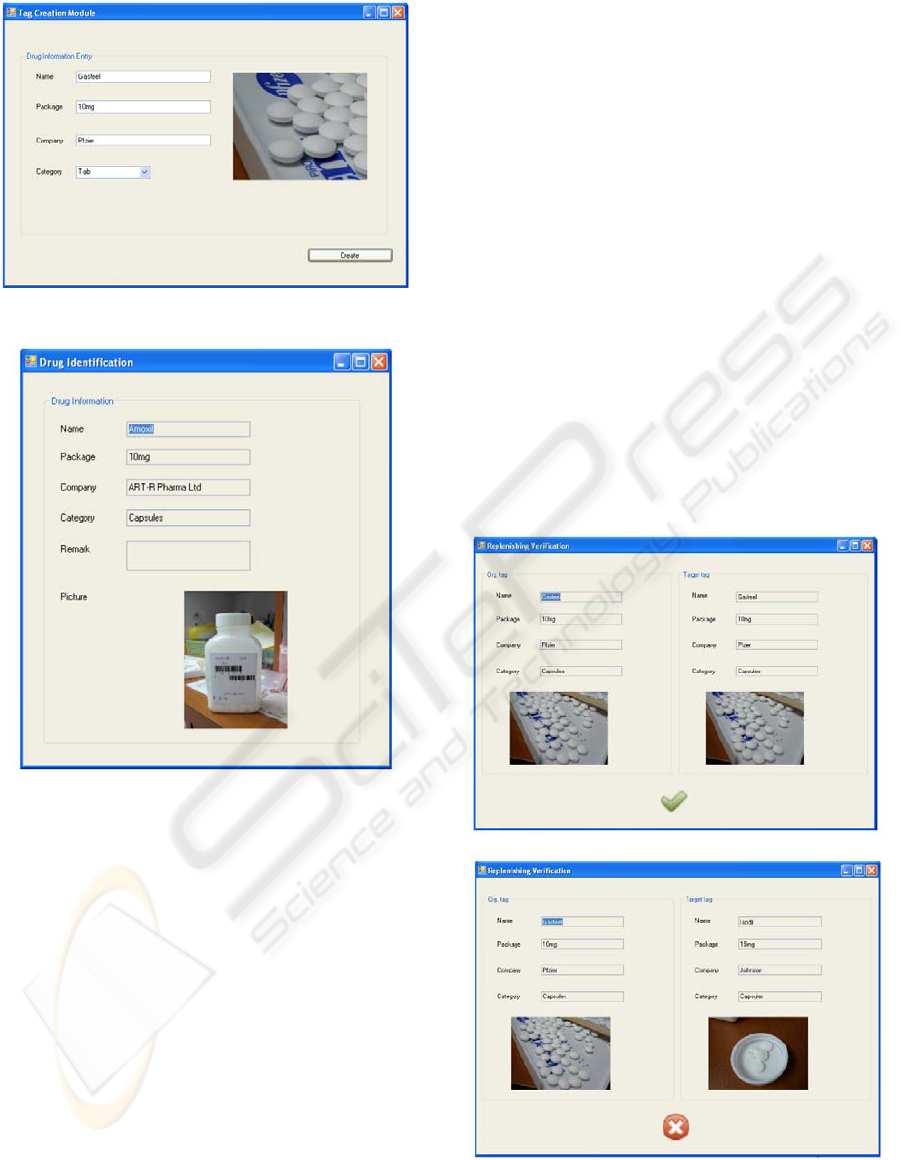
Figure 7: Screen Capture of Tag Creation Function.
Figure 8: Screen Capture of Drug Identification Function.
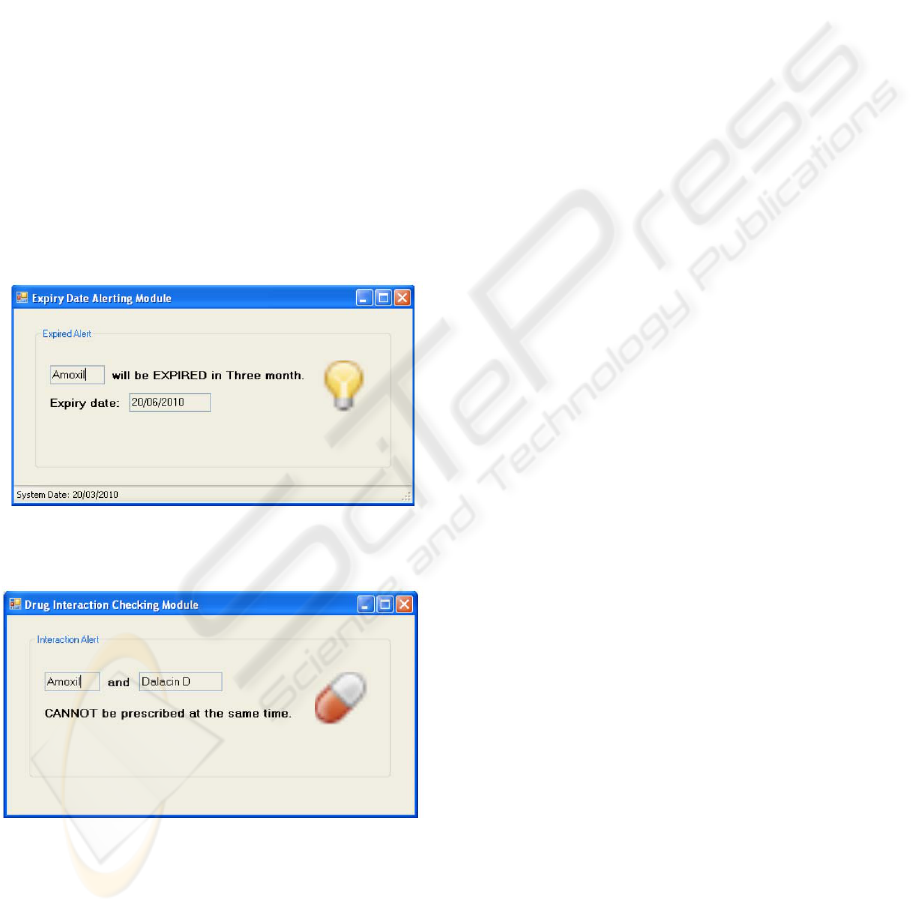
Expiry Date Alerting Function
As shown in Figure 10, this function
determines whether the drug is expired or not.
A large pop up alert message box is generated
to warn the medical workers about the expiry
date.
Drug-to-drug Interaction Checking Function
As shown in Figure 11, this function
determines whether the medicines dispensed to
the patient have interaction or not. Similar to
the Expiry Date Alerting Function, a large pop
up alert message box is generated to warn the
medical workers when interaction exists.
5 DISCUSSION
AND CONCLUSIONS
DMS can enrich, enliven and add variety to
traditional drug replenishing and dispensing
processes by using RFID technology. The following
are some of the significant impacts of DMS.
Originality, uniqueness and innovativeness
DMS is path breaking. Although some research
projects have investigated the feasibility of
implementing RFID in the dispensing process
(Lehmann and Kim, 2005), they overlook the
importance of drugs replenishing process.
Since the drugs replenishment process is the
initial stage to process the incoming drugs, if
mistakes are made in that process, medication
errors will eventually take place even though
the dispensing operation is correctly
performed. Moreover, in order to enhance the
innovativeness of DMS, special features like
drug images and interaction alert are added to
the DMS.
(a)
(b)
Figure 9: Screen Capture of Replenishing Verification
Function with (a) Matched tags (b) Unmatched tags.
AN RFID-BASED DRUG MANAGEMENT SYSTEM - A CASE IN MEDICAL ORGANIZATION
105
Practicality, User-friendliness, Extensibility &
Scalability
DMS provides users with information about
drugs immediately. By using DMS, they can
easily determine whether the drug has reached
its expiry date and it will interact with other
drugs. A real time alert (or pop up window) is
used to warn the users of abnormalities and
thus bring their awareness to the problematic
medicines. Furthermore, with the user-friendly
interface of DMS, users can easily distinguish
between drugs that look alike by the checking
system to avoid medication error. Furthermore,
the DMS applies an international standard and
a global unique serial number (i.e. EPC) to
identify drugs at item level that can be adopted
by different clinics or hospitals. When more
and more clinics and hospitals adopt the DMS,
it can be extended to pharmaceutical
manufacturers so that the whole drug supply
chain can be traced and tracked easily (ITU,
2005).
Figure 10: Screen Capture of Expiry Date Alerting
Function.
Figure 11: Screen Capture of Drug-Drug Interaction
Checking Function.
Social Responsibility
Traditionally, inspections are carried out only
when patients are found to be sick after taking
wrong medicine. However, with the help of
RFID, DMS improves the efficiency and
effectiveness of drug management. All the
problematic cases can be brought to the
attention of the operator (such as the nurse) in
real time. By adopting DMS, clinics can
adequately address social and environmental
concerns in their business operations. The
proposed system will assure drug safety
consistently. Thus, patient safety is enhanced
by reducing medication errors. In addition,
DMS speeds up the drug replenishing and
dispensing processes that help to reduce
operation expenses. This can drive the
stakeholders to scale up change throughout
their clinics.
ACKNOWLEDGEMENTS
The authors would also like to express their sincere
thanks to the Research Committee of the Hong Kong
Polytechnic University for providing the financial
support for this research work.
REFERENCES
Department of Health, 2008. Health Statistics , available
at: http://www.dh.gov.hk/. Accessed from 20 March
2010 14:03:40.
EPCglobal Inc., 2005. The EPCglobal Architecture
Framework, EPCglobal Final.
Fanberg, H., 2004. The RFID Revolution, Marketing
Health Services, Vol. 24, No. 3, pp. 43-44.
Huang, H. H., Ku, C. Y., 2009. A RFID Grouping Proof
Protocol for Medication Safety of Inpatient, Journal of
Medical Systems, Vol. 33, No. 6, pp. 467-474.
International Telecommunication Union (ITU), 2005. The
Internet of Things, ITU internet reports.
Kwok, S. K., Tsang, A. H. C., Ting, J. S. L., Lee, W. B.,
Cheung, B.C.F., 2008. An Intelligent RFID-based
Electronic Anti-Counterfeit System (InRECS) for the
Manufacturing Industry, In: Proceedings of the 17th
International Federation of Automatic Control (IFAC)
World Congress 2008, Seoul, Korea, July 6-11, 2008,
pp. 5482-5487.
Lehmann, C. U., Kim, G.R., 2005. Prevention of
Medication Errors, Clinics in Perinatology, Vol. 32,
No. 1, pp. 107-123.
McInnes, D. K., Saltman, D. C., and Kidd, M. R., 2006.
General practitioners’ use of computers for prescribing
and electronic health records: results from a national
survey, The Medical Journal of Australia, Vol. 185,
pp. 88–91.
Tam, K. W. T., Kwok, H. K., Fan. Y. M. C., Tsui, K. B.,
Ng, K. K., Ho, K. Y. A., Lau, K. T., Chan, Y. C., Tse,
C. W. C., Lau, C. M., 2008. Detection and prevention
INNOV 2010 - International Multi-Conference on Innovative Developments in ICT
106

of medication misadventures in general practice,
International Journal for Quality in Health Care, Vol.
20, No. 3, pp. 192-199.
Ting, S. L., Kwok, S. K., Tsang, A. H. C., Lee, W. B.,
2009. Critical Elements and Lessons Learnt from the
Implementation of an RFID-enabled Healthcare
Management System in a Medical Organization,
Journal of Medical Systems, DOI: 10.1007/s10916-
009-9403-5.
AN RFID-BASED DRUG MANAGEMENT SYSTEM - A CASE IN MEDICAL ORGANIZATION
107
