
A MORPHING TECHNIQUE TO ESTIMATE LUNG CANCER
DEFORMATION DUE TO BREATHING IN RADIOTHERAPIC
TREATMENT
Grazia Maria Pia Masselli, Luigi Battista, Sergio Silvestri
Faculty of Biomedical Engineering, University Campus Bio-Medico, via Alvaro del Portillo 200, 00128 Rome, Italy
Sara Ramella, Lucio Trodella
Radiotherapy and Oncology Unit, University Hospital, University Campus Bio-Medico
via Alvaro del Portillo 200, 00128 Rome, Italy
Keywords: Medical Image Detection, Acquisition, Analysis and Processing.
Abstract: A morphing technique aimed to correlate lung cancer patient’s chest cross circumference variations with
tumor morphology during quiet respiration is here described. Two CT slices corresponding to the same
tumor section are acquired at forced inspiration and forced expiration and correlated with chest
circumference values. An image sequence has been obtained by applying a linear morphing transformation.
Each image of the sequence has been associated with a chest circumferential value and a sequence subset
images corresponding to subject’s tidal volume has then been selected and compared with a CT slice
acquired at tidal volume. Images showing the minimum pixel differences with slice at tidal volume were
identified and associated with chest circumference values, allowing to estimate in which phase of the
breathing period the CT scan was carried out. CT exams in free-breathing and breath-hold conditions have
been conducted on a lung cancer patient in order to correlate the acquired slices with the variations of
patient’s chest circumference measured with a pneumatic strain gauge. The here described methodology
could allow to define the area to be irradiated during a particular phase of the breathing period, considering
the cancer area in the morphing simulation frame corresponding to this phase as target.
1 INTRODUCTION
Tumour motion due to respiration is an important
key issue for the development of accurate radiation
treatment of neoplasms located in lungs and
abdominal sites since, as it is well known, the
movement and deformation of tumors during the
breathing cycle affect not only the accuracy of CT
imaging but also the possibility of a successful
focused radiation treatment (Webb, 2006). Thus,
organ deformation during radiation delivery is a
geometric uncertainty that must be taken into
account in order to improve the quality and the
accuracy of radiotherapic treatment. The traditional
approach, according to ICRU (International
Commission on Radiation Units and Measurements)
Report 50 (ICRU Report 50, 1993), considers safety
margins around Gross Tumour Volume (GTV)
defined from a free-breathing CT scan: this method
estimates the extent of setup uncertainty and organ
motion and adds margins around a Clinical Tumour
Volume (CTV) to form a Planning Target Volume
(PTV). Several studies of the internal motion of the
tumour have been conducted based on the
hypothesis of rigid motion (Lujian et al, 1999, Wu et
al, 2004, Report 91 del AAPM Task Group 76,
2006) and different models of organ motion due to
respiration interpolating experimental data have
been proposed; moreover, it was observed that
abdominal organs motion due to respiration is well
correlated with diaphragm motion and it is
predominant in the craniocaudal direction. Some
authors (Lujian et al, 1999) have evaluated dose
delivered to the moving organ undergoing radiation
treatment as a function of the dose value predicted in
static case, considering an unidirectional movement
of the organ.The aim of focusing the dose within the
363
Maria Pia Masselli G., Battista L., Silvestri S., Ramella S. and Trodella L. (2010).
A MORPHING TECHNIQUE TO ESTIMATE LUNG CANCER DEFORMATION DUE TO BREATHING IN RADIOTHERAPIC TREATMENT.
In Proceedings of the Third International Conference on Bio-inspired Systems and Signal Processing, pages 363-366
DOI: 10.5220/0002591203630366
Copyright
c
SciTePress

region of interest minimizing the irradiation of
surrounding tissues, can be accomplished with
various methods such as, among others: 1)
continuous tracking systems, that allow the
irradiation of tumors while patient is breathing
normally –still in a research phase– (Murphy, 2007)
; 2) breath-hold techniques (Mageras and Yorke,
2004); 3) respiratory gated radiotherapy (Keall et al,
2006). Visual biofeedback techniques have also been
developed in order to reduce respiratory amplitude
(Masselli et al, 2009) and enhance respiration
reproducibility (Masselli et al, 2009, George et al,
2006)
and, in a preliminary way, for predicting
organ motion due to respiration during radiation
treatment (Briere et al, 2006, Venkat et al, 2008).
Recently, “morphing” techniques have been used in
a radiotherapy scenario (Deurloo et al, 2005), in
order to develop a method for the quantification of
tumor form variations in complex organs with
respect to the mean GTV obtained from elaboration
of CT slices acquired during free-breathing.
“Morphing” stands for “metamorphosing” and
indicates one of the first special digital effects used
in motion pictures and animations that allows to
transform a source image into a target image through
a seamless, fluid and gradual transition (Gomes et al,
1999). A morphing technique aimed to estimate lung
tumor deformation due to a lung cancer patient’s
breathing, in order to correlate patient’s chest
circumference variations with tumor morphology is
here proposed. The methodology allows to define
the area to be irradiated during a particular phase of
the breathing period, considering the cancer area in
the morphing simulation frame corresponding to this
phase as target.
2 METHODOLOGY
DESCRIPTION
A morphologic transformation is here used for
creating transitions between two morphological
configurations. Beyond the acquisition of a 2D CT
slice during patient freely breathing at tidal volume
(CT
TV
), in which lung tumour border line has
motion shadings because of breathing, CT images
have been acquired in breath-hold condition, i.e.
forced inspiration and forced expiration in order to
obtain static images of the lung tumour. These two
slices, approximately corresponding to the same
tumour section and referred to the two different
tumour configurations, called CT
MaxExp
and CT
MaxInsp
in the following, have been considered as the source
image and the target image for morphing sequence.
A pneumatic strain gauge (PSG) (Masselli et al,
2009) has been used in order to measure the
variations of patient’s chest cross section
circumference ∆C during the above reported CT
exams carried out in breath-hold and free-breathing
conditions. Values ΔC
MaxExp
and ΔC
MaxInsp
have been
measured in breath-hold conditions. During quiet
respiration the ∆C(t) has been measured, according
to patient’s respiratory pattern, obtaining an interval
of ∆C values comprised between ∆C
TVmin
and
∆C
TVmax
, which are the tidal volume chest
circumference at quiet expiration and inspiration end
respectively. The images CT
MaxInsp
and CT
MaxExp
have been loaded on a 2D morphing program
(http://www.stoik.com/) in order to generate a
simulation of the lung tumour deformation from
forced expiration to forced inspiration. After control
point allocation, the number of frames of the
morphing simulation has been set. Thus, the
program allowed to transform the markers on source
image in markers on target image. Marker
movement was regulated by a distortion curve: we
have considered a linear transformation. The quality
of simulation depends on the number and position of
chosen markers. In order to correlate ∆C values with
lung tumour morphology, the correspondence
between the number of each frame of morphing
simulation and ∆C values has been found
considering a n+2 frames sequence, where n is the
number of simulation frames generated by the
program and 2 are the source image CT
MaxInsp
(frame
0) and the target image CT
MaxExp
(frame n+1) of the
morphing transformation, that in turn refer to
ΔC
MaxExp
and ΔC
MaxInsp
measured values.The interval
between ΔC
MaxExp
and ΔC
MaxInsp
has been divided in
n+1 intervals having the same amplitude, in order to
associate a set of ∆C values with the corresponding
frame of morphing sequence. Thus, it has been
possible to calculate ΔC
MaxInsp
with the following
equation:
u)1n(CCC
MaxExp1nMaxInsp
⋅++==
+
ΔΔΔ
(1)
and, similarly, a generic value of chest
circumference ∆C
m
corresponding to the frame m of
morphing sequence:
umCC
MaxExpm
⋅+
=
Δ
Δ
(2)
It has been possible to associate the number of
each frame of morphing sequence with ∆C values
measured during CT scans. The sequence was
between the minimal and the maximal tumour
extension, so there were some frames of morphing
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
364

sequence describing lung tumour deformation during
free-breathing: by substituting the corresponding set
of ∆C values measured during CT exam in equation
(2), it has been possible to individuate the interval of
consecutive frames which described tumor motion
and deformation during tidal volume respiration.
Among these frames, there was the “best frame”,
which was the most similar to the tumour
configuration described by free-breathing CT slice
(CT
TV
) related to the same tumour section as CT
slices used for morphing simulation. This frame has
been found through the calculation of mean grey
levels of the difference image between each
simulation frame and the slice CT
TV
related to the
tumour configuration during free-breathing
acquisition: the frame whose difference image had
the smaller mean grey levels has been the most
similar to CT
TV
. By Eq. (2), ∆C value corresponding
to this best frame was calculated and compared with
the interval of ∆C
TV
values: in this way it was
possible to know in which phase of the breathing
period CT exam at tidal volume was carried out and
to define the target volume to be irradiated
corresponding to this phase.
3 RESULTS AND DISCUSSION
CT scans during free-breathing and in breath-hold
conditions have been conducted on a lung cancer
patient undergoing radiotherapic treatment. During
CT exams, patient’s chest circumference variations
have been measured with the PSG, obtaining
∆C
TVmin
=0 mm at the end of quiet expiration and
∆C
TVmax
=10 mm at the end of quiet inspiration on
average. ∆C
MaxInsp
=14 mm and ∆C
MaxExp
=-5 mm
during maximal inspiration and expiration,
respectively. We have considered ∆C
TVmin
as zero-
reference for ∆C measurements. Slices CT
MaxExp
and
CT
MaxInsp
acquired during CT carried out in forced
expiration and in forced inspiration respectively,
were associated with the measured ∆C
MaxExp
and
∆C
MaxInsp
values and have been loaded on the
morphing program for creating the interpolation
sequence. 56 markers have been placed on the
border line of the tumour (Fig. 1), a frame number
equal to 100 and a linear transformation have been
chosen for morphing sequence generation. By
substituting the above reported measured ∆C
TVmin
and ∆C
TVmax
in equation (2), the numbers of the
simulation frames corresponding to tumor
configuration during tidal volume breathing have
been calculated. Thus, the frames between 25 and 78
of the morphing simulation represented the minimal
and the maximal tumor extension during free-
breathing (Fig. 2). In order to verify the accuracy of
the method, the differences between simulation
frames and the image CT
TV
acquired during free-
breathing, after converting this slice from DICOM
format in 8 bit bitmap format with 256 grey levels
were calculated. In table 1 the numbers of simulation
frames, along with the corresponding mean grey
levels of the difference image are reported. From an
exam of table 1, it emerges that the frames more
similar to CT slice acquired during free breathing
are frames 25-60, because the corresponding
difference images have the smaller means of grey
levels. ∆C
values corresponding to these frames and
calculated by equation (2) were equal to 0-7 mm. By
comparing these values with the ∆C
TV
values
measured in the CT exam carried out during quiet
respiration, it emerges that the slice CT
TV
was
acquired approximately at the end of
expiration/beginning of inspiration, excluding the
inspiratory peak. Thus, in order to define the area to
be irradiated during each phase of tidal volume
respiration, the frames corresponding to the ∆C
interval between ∆C
TVmin
and ∆C
TVmax
have to be
considered. Target tumor can be delimited on these
frames, adding only a set up margin as safety
margin, since it allows for the uncertainties on
treatment reproducibility.
Figure 1: Markers placement on source image (forced
expiration) and on target image (forced inspiration).
Figure 2: Correspondence between simulation frames and
∆C values.
A MORPHING TECHNIQUE TO ESTIMATE LUNG CANCER DEFORMATION DUE TO BREATHING IN
RADIOTHERAPIC TREATMENT
365
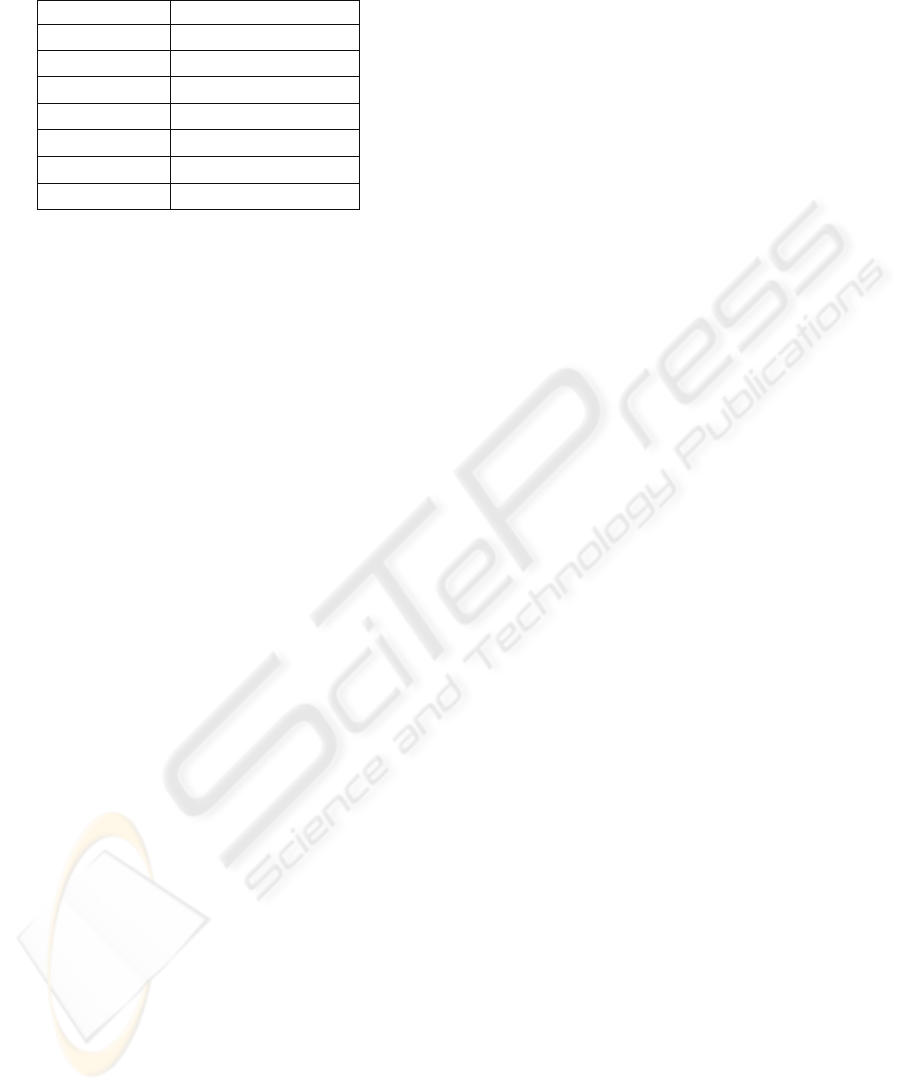
Table 1: Results of comparison between simulation frames
and CT slice acquired during free breathing.
Frame number Mean of grey levels
0-5 17
10-20 16
25-60 15
65-70 16
75-80 17
85-90 18
95-101 19
4 CONCLUSIONS
The here proposed technique allows to estimate the
lung tumor morphology during patient’s free-
breathing by acquiring CT slice at forced expiration
and forced inspiration. The technique could give an
important contribution for the improvement of
radiation treatment planning, always considering the
set up margin, which allows for the uncertainties on
treatment reproducibility. In order to define the area
to be irradiated during a particular phase of the
breathing period, the cancer area in the simulation
frame corresponding to this phase has to be
considered as target: this allows the absence of
motion shadings. The present work represents only a
first stage study which could allow to deliver a high
dose to the tumour while minimizing the dose
delivered to the surrounding healthy tissue, though
further researches with more subjects are still needed
in order to test the accuracy of the presented
methodology.
REFERENCES
Webb, S. (2006) ‘Motion effects in (intensity modulated)
radiation therapy: a review’, Phys. Med. Biol., vol. 51,
pp. R403-R425.
ICRU Report 50 (1993) Available:
http://www.aitro.it/public/Crs100icru.pdf
[20 Mar 2009].
Lujian, A. E., Larsen, E. W., Balter, J. M., Haken, R. K.
T. (1999) ‘A method for incorporating organ motion
into 3D dose calculations’, Med. Phys., vol. 26, pp.
715-720.
Wu, H., Sharp, G. C., Saltzberg, B., Kaeli, D., Shirato, H.,
Jang, S. B. (2004) ‘A finite state model for respiratory
motion analysis in image guided radiation therapy’,
Phys. Med. Biol., vol. 49, pp. 5357-5372.
Report 91 del AAPM Task Group 76 (2006) ‘The
management of respiratory motion in radiation
oncology’, American Association of Physicists in
Medicine.
Murphy, M. J., Balter, J., Balter, S., BenComo, J. A. Jr,
Das, I. J., Jiang, S. B., Ma, C. M., Olivera, G. H.,
Rodebaugh, R. F., Ruchala, K. J., Shirato, H., Yin, F.
F. (2007) ‘The management of imaging dose during
image-guided radiotherapy: report of the AAPM Task
Group 75’, Med. Phys., vol. 34, pp. 4041-4063.
Mageras, G. S., Yorke, E. (2004) ‘Deep inspiration breath
hold and respiratory gating strategies for reducing
organ motion in radiation treatment’, Semin. Radiat.
Oncol., vol. 14, pp. 65-75.
Keall, P. J., Mageras, G. S., Balter, J. M., Emery, R. S.,
Forster, K. M., Jiang, S. B., Kapatoes, J. M., Low, D.
A., Murphy, M. J., Murray, B. R., Ramsey, C. R., Van
Herk, M. B., Vedam, S. S., Wong, J. W., Yorke, E.
(2006) ‘The management of respiratory motion in
radiation oncology report of AAPM Task Group 76’,
Med. Phys., vol. 33, pp. 3874-3900.
Masselli, G. M. P., Silvestri, S., Ramella, S., Trodella, L.
(2009) ‘Design and evaluation of a methodology to
perform personalized visual biofeedback for reducing
respiratory amplitude in radiation treatment’, Med.
Phys., vol. 35, no. 5, pp. 1467-1472.
George, R., Chung, T. D., Vedam, S. S., Ramakrishnan,
V., Mohan, R., Weiss, E., Keall, P. J. (2006) ‘Audio-
visual biofeedback for respiratory-gated radiotherapy:
impact of audio instruction and audio-visual
biofeedback on respiratory-gated radiotherapy’, Int. J.
Radiat. Oncol. Biol. Phys., vol. 65, pp. 924-933.
Briere, T., Jhaveri, P., Krishnan, S., Crane, C., Balter, P.,
Gillin, M., Mohan, R., Beddar, A. (2006) ‘SU-FF-T-
114: Breath Coaching with Visual Feedback for End-
Expiratory Gated Radiotherapy’, Med. Phys., vol. 33,
p. 2075.
Venkat, R. B., Sawant, A., Suh, Y., George, R., Keall, P.
J. (2008) ‘Development and preliminary evaluation of
a prototype audiovisual biofeedback device
incorporating a patient-specific guiding waveform’,
Phys. Med. Biol., vol. 53, pp. N197-N208.
Deurloo, K. E. I., Steenbakkers, R. H. M., Zijp L. J. et al.
(2005) ‘Quantification of shape variation of prostate
and seminal vesicles during external beam
radiotherapy’, Int. J. Radiation Oncology Biol. Phys.,
vol. 61, pp. 228-238.
Gomes, J., Darsa, L., Costa, B., Velho, L. (1999)
‘Warping and Morphing of Graphical Objects’,
Morgan Kaufmann Editor.
http://www.stoik.com/ [25 Mar 2009].
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
366
