
IMMUNOSENSORS FOR ATRAZINE DETECTION
IN RED WINE SAMPLES
Enrique Valera, Ángel Rodríguez
Micro and Nano Technologies Group (MNTg), Departament d’Enginyeria Electrònica
Universitat Politècnica de Catalunya,C/. Jordi Girona 1-3 Campus Nord, Mòdul C4, Barcelona 08034, Spain
Javier Ramón-Azcón, Francisco J. Sanchez, M.-Pilar Marco
Applied Molecular Receptors Group (AMRg), IIQAB-CSIC
CIBER of Bioengineering, Biomaterials and Nanomedicine, Jordi Girona 18-26, 08034 Barcelona, Spain
Keywords: Immunosensor; Interdigitated μ-electrodes, Atrazine; Impedance spectroscopy, Conductive measurements,
Wine matrix effect, Food safety.
Abstract: Two novel immunosensors, one impedimetric and other one conductimetric, for atrazine detection in red
wine samples have been developed. Impedimetric immunosensor is based on an array of interdigitated μ-
electrodes (IDµEs) and bioreagents specifically developed to detect this pesticide. Conductimetric
immunosensor incorporates additionally gold nanoparticles. Bioreagents were covalently immobilized on
the surface of the electrodes (interdigital space). In both cases the biochemical determination of atrazine is
possible without any redox mediator. For the case of the impedimetric immunosensor, the detection method
is based on impedimetric measurements (in a wide range of frequencies), whereas in the case of the
conductimetric immunosensor the detection method is based on conductimetric measurements (DC
measurements).The potential of the impedimetric immunosensor to analyze atrazine in complex sample
matrices, such as red wine, have been evaluated. This immunosensor can detect atrazine with limits of
detection in the order sub-ppb, far below the maximum residue level (MRL) (50 µg L
−1
) established by
European Union (EU) for residues of this herbicide in the wine grapes.
1 INTRODUCTION
In the recent years, modern chemical analysis has
been revolutionized by the electrochemical
biosensors because of their accuracy, technical
simplicity, high efficiency, possibility of portability
and miniaturization, and because they offer fast
(instantaneous) response times, allow a rapid and
permanent control and a direct transduction of the
biomolecular recognition event into electronic
signals (Murphy, 2006; Pumera, 2007; Wang, 2006).
An important disadvantage of the
electrochemical sensors is that the impedance
changes due to biomolecular recognition are
generally very small and it can not reach the
necessary detection limits required by the
legislation. However, this disadvantage can be
solved by applying techniques such as
electrochemical impedance spectroscopy (EIS) or
including labels that amplify the signal.
By means of EIS is possible to record
information on biorecognition events, occurring at
the electrode surfaces, inducing impedance changes
(Guan et al., 2004; Katz and Willner, 2003), that can
be directly measure, allowing the development of
label-free biosensing devices.
On the other hand, many types of labels are used
to amplify biorecognition events. Between the
labels, the gold nanoparticles are some of the most
recently used (Pumera, 2007; Zhang, 2007), because
its unique properties at nanoscale dimension.
In this work we report the description of two
immunosensors for atrazine detection. One
immunosensor is label-free, whereas the other one is
labelled with gold particles (40 nm). In the case of
the label-free immunosensor, impedimetric
measurements (impedance spectroscopy) are used as
detection method. In the second case, due the
presence of the gold particles, conductimetric
measurements (DC measurements) could be used.
226
Valera E., Rodríguez Á., Ramón-Azcón J., J. Sanchez F. and Marco M. (2009).
IMMUNOSENSORS FOR ATRAZINE DETECTION IN RED WINE SAMPLES .
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 226-230
DOI: 10.5220/0001542002260230
Copyright
c
SciTePress

In order to demonstrate the applicability of the
devices developed, wine production has been
selected as scenario for the proof-of-concept study.
Reasons for this selection are because wine is a high
value product (with a great economic relevance in
EU) and because their strong matrix effect. In fact, if
the sensors are demonstrated to red wine samples,
their use can be extrapolated to many other matrices.
Likewise, we will prove that the impedimetric
immunosensor can respect the Maximum Residue
Level (MRL) established by EU (European Union)
for residues of this herbicide in wine grapes (50 µg
L
-1
).
2 EXPERIMENTAL
2.1 Instrumentation
Impedimetric and conductive measurements were
carried out at room temperature in a probe station
(Faraday cage) KARL SUSS. Impedance analyses
were performed using an Agilent 4294A Precision
Impedance Analyzer and conductive measurements
were performed using an Agilent 4156C
Semiconductor Parameter Analyzer. The
competitive curves were analyzed with a four-
parameter logistic equation using the software
SoftmaxPro v2.6 (Molecular Devices) and GraphPad
Prism version 4.00 for Windows (GraphPad
Sofware, San Diego California USA). Data shown
correspond to the average of at least three replicates
per concentration of atrazine.
2.2 Arrays of Interdigitated
μ-electrodes
The immunosensors developed are based on a two
coplanar non-passivated interdigitated metallic μ-
electrodes. Thin Au/Cr (200 nm thickness)
interdigitated μ–electrodes with 10 µm pitch were
patterned on a Pyrex 7740 glass substrate (purchased
from Präzisions Glas&Optik GmbH, 0.7 mm (±0.05)
thickness). For the immunosensor measurements,
electrode arrays were constructed consisting on six
IDµEs organized on a 0.99 cm
2
area. The Pyrex
substrate was first cleaned using absolute ethanol.
Following metal deposition was performed by
sputtering and the interdigitated μ-electrodes were
patterned by a photolithographic metal etching
process. The chromium layer, much thinner than the
gold layer, was deposited prior the gold to improve
adhesion to the Pyrex substrate.
2.3 Immunosensors Functionalization
Biofunctionalization with 2d-BSA was done
selectively on the surfaces of the gold electrodes.
The chemical recognition layer was covalent
immobilized on the interdigital space. For this
purpose the array of IDµEs were treated as follows.
2.3.1 Surface Cleaning
Before functionalization, the IDµEs samples were
first cleaned with a combination of ethanol:Mili-Q
water 70:30, absolute ethanol absolute, piranha
solution and absolute ethanol.
2.3.2 Surface Activation
After the pre-treatment explained above, surface
activation took place in two steps to modify
selectively the gold electrodes and the Pyrex
substrate.
Activation of gold surfaces was readily and
specifically performed using thiol-chemistry. N-
acetylcysteamine was used to cover the gold
electrodes and to protect the sensor from undesired
non-specific absorptions. Thus, the surface texture
of the IDµE defines the template for deposition of
layers, since the gold fingers have been deposited on
a solid support such as glass with the necessary
controlled geometry. In a second step, the Pyrex was
derivatized with 3-glycidoxypropyl trimethoxysilane
(GPTS). The epoxy group provided the necessary
reactivity for further attachment of the bioreagents
through a nuchelophylic attack of functional groups
of the biomolecule such as the amino groups of the
lysine residues (Luzinov, 2000). The immunosensor
surface functionalization is schematically shown in
Figure 1.
2.3.3 Antigen Immobilization
Covalent immobilization of the pesticide antigen 2d-
BSA was performed on the interdigitated μ-
electrodes surface via the amino groups of the lysine
residues by reaction with the epoxy groups of the
surface.
2.4 Competitive Assay
The assay of detection, common for both
immunosensors, relies on the immunochemical
competitive reaction between the atrazine residues
and the immobilized antigen on IDµEs for a small
amount of the specific antibody (primary antibody).
The detection of a small number of molecules of
IMMUNOSENSORS FOR ATRAZINE DETECTION IN RED WINE SAMPLES
227

atrazine is performed under competitive conditions
involving the competition between the free pesticide
(analyte) and a fixed amount of coated antigen for a
limited amount (low concentration) of primary
antibody (Ab
1
). At the end of the reaction the
amount of Ab
1
captured on the IDµE surface and
hence the free antigen (analyte) is determined.
Finally, and only in the case of the
conductimetric immunosensor, a secondary labelled
with gold antibody (Ab
2
) is captured in order to
amplify the conductive signal. The immunosensor
assay is schematically shown in Figure 2.
2.5 Impedance Measurements
Impedance measurements were carried out at room
temperature. No redox mediator was used in the
devices presented in this work. The two electrodes
were covered by a diluted PBS solution with a
conductivity of 1.6 µS cm
−1
and connected to the
input of an Agilent 4294A Precision Impedance
Analyzer by means of standard probe tips.
Measurements were taken in the 40 Hz to 1MHz
frequency range using 0V of polarization potential
and a modulation voltage of 25mV amplitude. All
impedance measurements were performed in a
Faraday cage.
2.6 Conductance Measurements
As for impedance measurements, the conductance
measurements were also carried out at room
temperature without any redox mediator and in a
Faraday cage. The two electrodes were covered by a
diluted PBS solution with a conductivity of 1.6 µS
cm
−1
and connected to the input of an Agilent 4156C
Semiconductor Parameter Analyzer by means of
standard probe tips. Conductivity was measured to
+25 mV, from +22.5 to +27.5 mV sweep bias. These
conductive measurements were performed after the
incubation step of the secondary antibody labelled
with gold.
3 ATRAZINE DETECTION
In the case of the impedimetric immunosensor, the
quantitative tool that seems adequate to provide
sensitivity graphs is the impedance measurement in
a wide frequency range and the fitting of the Nyquist
plots of impedance spectra to an equivalent circuit.
Then, the atrazine concentration should finally
be related to the values of at least some of the
parameters of the equivalent circuit. The equivalent
circuit used was previously reported (Valera, 2007)
and the resistance of the solution (Rs) was chosen as
parameter of analysis.
Interdigitated μ-electrode (IDµE)
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
IDµEs protection with N-Acetylcysteamine
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
inter-digits space functionalization with GPTS
Si
CH
2
OO
O
CH
2
CH
2
O
CH
2
HC
H
2
C
O
Si
CH
2
OO
O
CH
2
CH
2
O
CH
2
HC
H
2
C
O
Si
CH
2
OO
O
CH
2
CH
2
O
CH
2
HC
H
2
C
O
Si
CH
2
OO
O
CH
2
CH
2
O
CH
2
HC
H
2
C
O
Figure 1: Schematic diagram of: i) protection of
interdigitated μ-electrodes with N-acetylcysteamine; and
ii) immunosensor surface functionalization with GPTS.
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
coated antigen
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
NH
2
SO
2
NH
N
primary
specific
antibody
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
O
Si
CH
2
O
O
CH
2
CH
2
HC
H
2
C
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH
H
2
C
CH
2
S
O
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH2
SO
2
NH
N
NH
2
SO2NH
N
NH
2
SO2NH
N
NH2
SO
2
NH
N
secondary
antibody
labelled wit
h
gold
nano
p
articles
Figure 2: Schematic diagram of the complete assay system
performed on the IDµEs for the immunosensors
developed.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
228
For the conductimetric immunosensor, the
measurement of the conductance after the capture of
the secondary labelled with gold antibody could be
used as detection method, as it was previously
demonstrated using PBS in the competitive assay
(Valera, 2008). Then, the atrazine concentration
would be related to the amount of gold nanoparticles
present on the immunosensor.
The impedimetric response of the immunosensor
in red wine is shown in Figure 3.
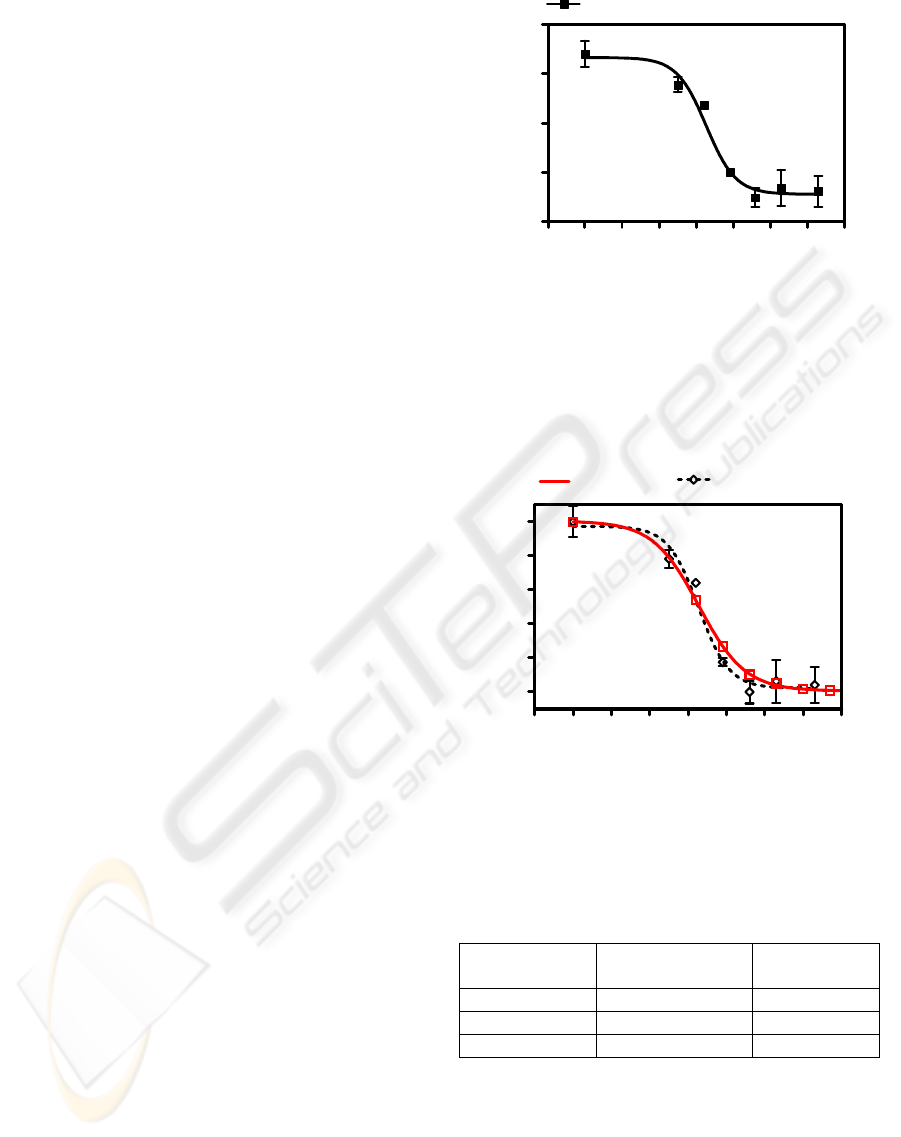
To validate the sensing approach, our
immunosensors were also characterized by means of
chemical affinity methods. In Figure 4 the results
obtained from the immunosensor are compared with
the results of an ELISA assay on the same IDµEs
devices but using a chemically raised colorimetric
signal. In order to comparatively show the
immunosensor performance, in Figure 4 the
normalized values of the change in the Rs as a
function of the atrazine concentration as well as the
normalized results of the ELISA assay are plotted.
It is important to remark that the ELISA assay
performed on the IDµE device show a similar
analytical profile that the obtained using microtitrer
plates. Therefore, we could be confident that our
immunosensor really reflects the selective binding
event. The more important analytical features of the
atrazine assays (immunosensors and ELISA) are
shown in Table 1.
As it can be seen in Table 1, using the
impedimetric immunosensor is possible to detect
atrazine in sub-ppb concentrations (0.19 µg L
−1
).
These good results are directly related to the
advantages of the impedimetric device presented
such as the use of IDµEs and the competitive assay
based on the antibodies variation.
Comparing these results with the recent
literature, the impedimetric immunosensor presented
in this work have been demonstrated to be more
sensitive that other biosensors based on
impedimetric methods for the atrazine detection
(Helali, 2006; Hleli, 2006; Fredj, 2008), specially
taking into account that our immunosensor have
been tested using red wine samples.
10
-4
10
-3
10
-2
10
-1
10
0
10
1
10
2
10
3
10
4
400
800
1200
1600
2000
Impedimetric immunosensor
Red wine
[Atrazine, μg L
-1
]
Δ
Rs (
Ω
)
Figure 3: Impedimetric response when the immunosensor
are used to detect atrazine. Reprinted from Biosensors and
Bioelectronics, 23, J. Ramón-Azcón et al., An
impedimetric immunosensor based on interdigitated
microelectrodes (IDµE) for the determination of atrazine
residues in food samples, 1367–1373, (2008), with
permission from Elsevier.
10
-4
10
-3
10
-2
10
-1
10
0
10
1
10
2
10
3
10
4
0
20
40
60
80
100
Impedimetric
Immunosensor
ELISA
[Atrazine, μg L
-1
]
Normalized signals
Figure 4: Normalized calibration curves of the optimized
atrazine immunoassay and the impedimetric
immunosensor presented. Measures were taken in diluted
PBS solution.
Table 1: Features of the atrazine assays in red wine
samples.
Impedimetric
Immunosensor
ELISA
IC
50
, µg L
-1
1.876±0.23 1.93±0.02
LOD, µg L
-1
0.19 0.09
R
2
0.86 0.99
Reprinted from Biosensors and Bioelectronics, 23, J.
Ramón-Azcón et al., An impedimetric immunosensor
based on interdigitated microelectrodes (IDµE) for the
determination of atrazine residues in food samples, 1367–
1373, (2008), with permission from Elsevier.
IMMUNOSENSORS FOR ATRAZINE DETECTION IN RED WINE SAMPLES
229

4 CONCLUSIONS
Two immunosensors, one impedimetric and other
one conductimetric, for the atrazine detection in red
wine samples have been developed. Both devices are
based on an array of IDµEs and in bioreagents
specifically developed. Impedimetric immunosensor
is able to detect atrazine in red wine at sub-ppb
concentrations, far below the Maximum Residue
Level (MRL, 50 µg L
−1
) required by EC. However,
this result could be improved using the
conductimetric device, which includes secondary
antibodies labelled with gold particles that increase
the conductive signal.
ACKNOWLEDGEMENTS
This work has been partially supported by the
Ministry of Science and Technology (Contract
number TEC2007-67081) and FEDER funds. The
MNT group is a consolidated Grup de Recerca de la
Generalitat de Catalunya since the year 2001
(expedient 00329). The AMR group is a
consolidated Grup de Recerca de la Generalitat de
Catalunya and has support from the Departament
d’Universitats, Recerca i Societat de la Informació la
Generalitat de Catalunya (expedient 2005SGR
00207).
REFERENCES
Fredj, H. Ben., Helali, S., Esseghaier C., Vonna L., Vidal,
L., Abdelghani, A. Labeled magnetic nanoparticles
assembly on polypyrrole film for biosensor
applications. Talanta 75 (2008) 740-747.
Guan, J.G., Miao, Y.Q. Zhang, Q.J. Impedimetric
Biosensors. Journal of Bioscience and Bioengineering
97 (2004) 219–226.
Helali, S., Martelet, C., Abdelghani, A., Maaref, M.A.,
Jaffrezic-Renault, N. A disposable immunomagnetic
electrochemical sensor based on functionalised
magnetic beads on gold surface for the detection of
atrazine. Electrochim. Acta 51 (2006) 5182–5186.
Hleli, S., Martelet, C., Abdelghani, A., Burais, N.,
Jaffrezic-Renault, N. Atrazine analysis using an
impedimetric immunosensor based on mixed
biotinylated self-assembled monolayer, Sensors and
Actuators B 113 (2006) 711–717.
Katz, E., Willner, I. Probing Biomolecular Interactions at
Conductive and Semiconductive Surfaces by
Impedance Spectroscopy: Routes to Impedimetric
Immunosensors, DNA-Sensors, and Enzyme
Biosensors. Electroanalysis 15 (2003) 913–947.
Luzinov, I., Julthongpiput, D., Liebmann-Vinson, A.,
Cregger, T., Foster, M.D., Tsukruk, V.V., Epoxy-
Terminated Self-Assembled Monolayers: Molecular
Glues for Polymer Layers. Langmuir 16 (2000) 504-
516.
Maggio, E.T., 1981. Enzyme-Immunoassay, CRC Press,
Florida.
Murphy, L., Biosensors and bioelectrochemistry. Current
Opinion in Chemical Biology 10 (2006) 177–184.
Pumera, M., Sánchez, S., Ichinose, I., Tang, J.
Electrochemical nanobiosensors. Sensors and
Actuators B 123 (2007) 1195–1205.
Valera, E., Ramón-Azcón, J., Rodríguez, A., Castañer, L.-
M., Sanchez-Baeza, F.-J., Marco, M.-P. Impedimetric
immunosensor for atrazine detection using
interdigitated μ-electrodes (IDµE’s). Sensors and
Actuators B 125 (2007) 526–537.
Valera, E., Ramón-Azcón, J., Sanchez-Baeza, F.-J.,
Marco, M.-P, Rodríguez, A.. Conductimetric
immunosensor for atrazine detection based on
antibodies labelled with gold nanoparticles. Sensors
and Actuators B (2008) 95-103.
Wang, J. Electrochemical biosensors: Towards point-of-
care cancer diagnostics. Biosensors and Bioelectronics
21 (2006), pp. 1887–1892.
Zhang, S.-B., Wu, Z.-S., Guo, M.-M., Shen, G.-L. Yu, R.-
Q. A novel immunoassay strategy based on
combination of chitosan and a gold nanoparticle label.
Talanta 71 (2007) 1530–1535.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
230
