
A BIO-INSPIRED CONTRAST ADAPTATION MODEL AND ITS
APPLICATION FOR AUTOMATIC LANE MARKS DETECTION
Valiantsin Hardzeyeu and Frank Klefenz
Fraunhofer Institute for Digital Media Technology, Langewiesener str. 22, 98693, Ilmenau, Germany
Keywords: Retina, contrast adaptation, visual perception, fuzzy-like sets, lane marks detection.
Abstract: Even in significant light intensity fluctuations human beings still can sharply perceive the surrounding
world under various light conditions: from starlight to sunlight. This process starts in the retina, a tiny
tissue of a quarter of a millimeter thick. Based on retinal processing principles, a bio-inspired computational
model for online contrast adaptation is presented. The proposed method is developed with the help of the
fuzzy theory and corresponds to the models of the retinal layers, their interconnections and
intercommunications, which have been described by neurobiologists. The retinal model has been coupled in
the successive stage with the Hough transformation in order to create a robust lane marks detection system.
The performance of the system has been evaluated with the number of test sets and showed good results.
1 INTRODUCTION
Human beings get a significant part of information
through the visual perception system which consists
of the retina, the visual nerve and the visual cortex in
the midbrain. The retina in this sequence plays the
role of a pre-processor and reduces the information
delivered to the visual cortex. In this paper we like
to point out how the retina adapts the intensity
fluctuations that appear in the real-life situations and
describe a method for the contrast adaptation with
the help of the fuzzy–like sets.
According to the work that is presented in
(Hubel, 1995) and (Masland, 2001), the retina is a
part of the brain, which has been separated from it
during the early stages of development, but having
kept the connections to the brain through the optic
nerve. Five different types of cells form the retina:
photoreceptors, horizontal cells, bipolar cells,
amacrine cells and ganglion cells. They all are
organized in a layered structure and the visual data
flows from the upper layer (photoreceptors) to the
lower layer (ganglion cells) in a parallel manner.
Their interconnections are well described in (Hubel,
1995). Among the other important functions of the
retina, like edge extraction and motion detection
(Olveczky et al., 2003), the real-time
implementation of the contrast adaptation seems to
be important for almost all image processing and
robotic projects.
As described in (Smirnakis et al., 1997), the
contrast adaptation process begins in the lower
layers of the retina (amacrine and ganglion cells)
and allows the retinal neurons to use their dynamic
range more efficiently. The recovery time of the
visual system after changing the ambient intensity is
several seconds (Baccus and Meister, 2002) and in
the (Solomon et al., 2004) were reported that when
the mean intensity increase, the retina becomes less
sensitive. These biological principles for the contrast
adaptation were taken as a basis for the
development.
As it pointed out in (Wilson, 1993), the contrast
adaptation process which takes place in the retina
can be described with help of differential equations.
As an alternative, we found a method to describe
this non-linear process with fuzzy-like sets and
coupled the system with the Hough transform for
lane marks detection.
2 RETINA MODEL FOR
CONTRAST ADAPTATION
Five different layers (three vertical and two
horizontal) build up the retina. Vertical layers are
presented by photoreceptors (rods and cones),
bipolar and ganglion cells and form the direct
pathway of the visual data flow. Horizontal layers of
the retina are presented by the horizontal and
513
Hardzeyeu V. and Klefenz F. (2008).
A BIO-INSPIRED CONTRAST ADAPTATION MODEL AND ITS APPLICATION FOR AUTOMATIC LANE MARKS DETECTION.
In Proceedings of the First International Conference on Bio-inspired Systems and Signal Processing, pages 513-520
DOI: 10.5220/0001065705130520
Copyright
c
SciTePress
amacrine cells and, together with the vertical layers
form the indirect pathway. Both paths are needed for
the sufficient visual information pre-processing and
for forming the signals to the inner brain.
2.1 Two Layers, Three Processing
Tasks
The cells in the inner retina are organized in a
parallel manner and build together a highly
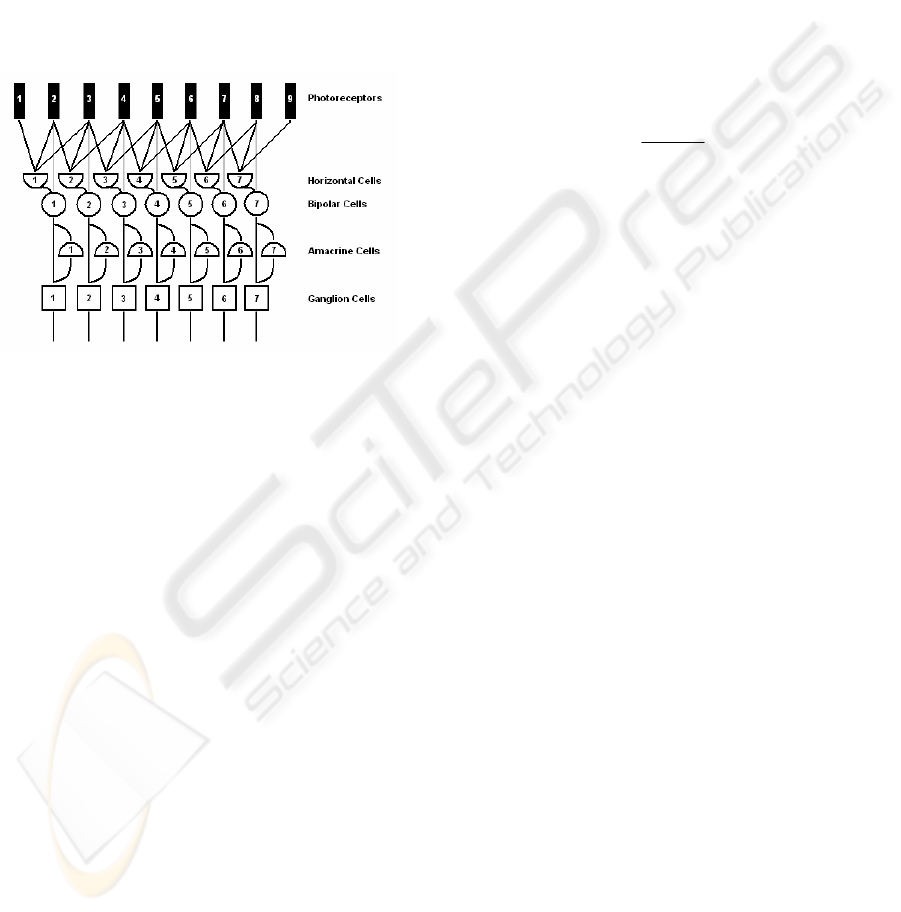
distributed structure. In fig. 1 the digital
representation of all five retinal cells and their
interconnections is shown.
Figure 1: Digital representation of the retinal layers.
All retinal cells can be divided into two
processing layers by their functionality. The first
layer is presented by the photoreceptors, horizontal
and bipolar cells, and performs the edge extraction
(Hubel, 1995), (Olveczky et al., 2003), while the
second layer, which is formed by the amacrine and
ganglion cells, performs among other tasks the local
motion detection and the direction of movement
estimation (Masland, 2001), (Berry II et al., 1999).
Since the contrast adaptation also begins in the
lower retinal layers (amacrine and ganglion cells), it
is important to understand the responses from the
higher processing layers (photoreceptors – bipolars).
2.2 Modelling of the Bipolar Cells
Response
The processing on the first layers starts from
photoreceptors that sense the incoming light. Some
of the photoreceptors are activated by the presence
of light while others are activated when they do not
detect light. All of them are arranged in a circular
way so that one type is surrounded by other types
(center–surround organization). In this paper we use
the ‘on–center’ surrounding organization scheme
(Hubel, 1995).
On the next level, the horizontal cells get their
input from the photoreceptors. They play a very
important role in reducing the amount of information
that is given to the inner brain and represent an
additional mechanism which helps to adjust the
retina response to the overall level of illumination.
Their task is to measure the illumination across a
broad region of photoreceptors and pass the average
value further to the next level. Such calculation can
be represented by Equation 1, where P
k
is the output
of each photoreceptor that is connected to a
horizontal cell H
i
; n is the number of inputs of a
certain horizontal cell.
n
H
n
k
k
i
p
∑
=
=
1
(1)
On the third level, the bipolar cells get their
inputs from the center photoreceptors directly and
from the surrounding photoreceptors indirectly
through the horizontal cells. These two inputs build
the receptive field of each bipolar cell.
The function of the bipolar cell involves a
subtraction mechanism: it subtracts the value of the
horizontal cell H from the value which is received
from the center photoreceptors. Thus, the output of
each bipolar cell B
i
can be represented by the
Equation 2, where B
i1
is the input from the
photoreceptors and the B
i2
– is the input from the
horizontal cell H
i
.
B
i
= B
i1
– B
i2
(2)
The output of each bipolar cell forms the
response from the whole receptive field and in this
stage retina performs the edge extraction function
(Olveczky et al., 2003). As it is known from the
classical theory for image processing (Shapiro,
2001), the edge detection operators highlight the
boundaries between regions of different intensities.
This is, naturally, how human beings perceive the
perimeter of an object, when it differs by its
intensity from the background. In fig. 2 the stimuli
with a step-change border and a simplified model of
the first stages of the retina are presented. Here we
assume that each photoreceptor corresponds to a
single pixel in the image and each bipolar cell B is
driven by the receptive field which is constructed by
three photoreceptors – one for the center response
and two for the surrounding. The receptive fields of
the different bipolar cells overlap each other (Hubel,
1995) and, thus, each photoreceptor is fed not only
to the single bipolar cell, but to a number of bipolar
cells.
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
514
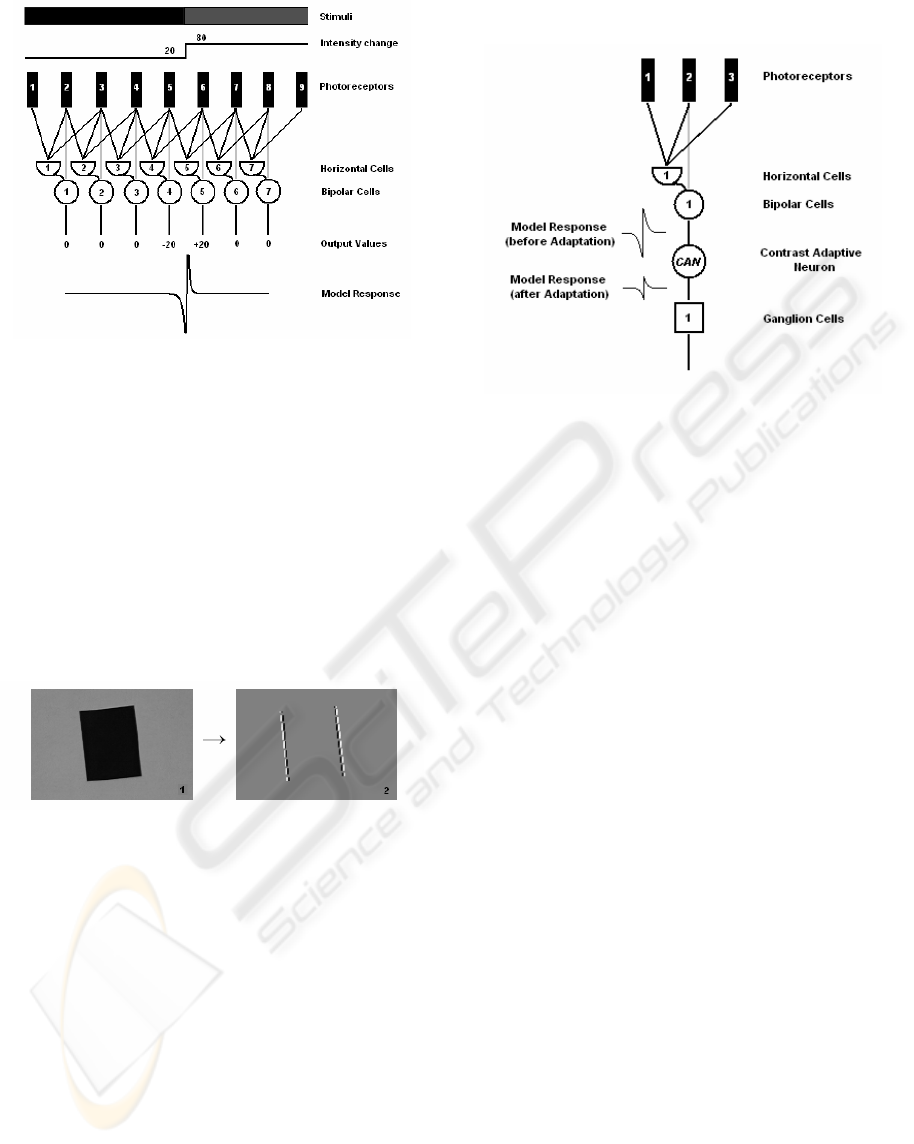
Figure 2: The model and its edge response.
In this example the stimuli change their
intensities between the receptors 5 and 6 from 20 to
80. The model’s response on the step-change border
can be presented by the activities of the two peaks
(negative and positive) exactly at the border between
the two regions. The absolute values of the peaks are
equal, but differ by the sign. Such bio-inspired edge
extraction technique called zero-crossing has been
confirmed by Marr (Marr, 1982) while investigating
the neurobiological background of vision. Fig. 3
shows the response of the bipolar cells at vertical
edges.
Figure 3: The stimuli and the bipolar cell’s response at
vertical edges.
The bipolar cells are fed to the amacrine and
ganglion cells, but first the signal from the bipolar
cell reaches the Contrast Adaptive Neuron.
2.3 Contrast Adaptive Neuron and its
Function
According to (Smirnakis et al., 1997), when the
mean intensity of ambient light increases, the retina
becomes less sensitive. This process is organized
with the help of the contrast adaptive neuron (CAN),
which is located just after the bipolar cells and
serves to adjust the input activity of the ganglion
cells in order to use their dynamic range more
efficiently. In fig. 4 the simplified model of the
receptive field for a single ‘on–center’ ganglion cell
with a CAN is presented.
Figure 4: The model of the ganglion cells receptive field
with CAN.
For fig. 4 we assume that the response generated
by the bipolar cell lies above the ganglion cells
dynamic range and the CAN brings the bipolar cell
response back to the dynamic range of the ganglion
cell by changing its amplitude value. However, the
retina adapts the high and low intensities differently.
When the contrast changes from low to high
(positive contrast change, e.g., going from normal
light room conditions to the strong sun light at
midday), in the first tens of a second the retina
decreases the sensitivity of CAN dramatically, that
results in a quick decrease of the ganglion cell’s
activity. Such first step of the adaptation process is
called “Fast adaptation” and helps to bring the
ganglion cell input nearly to its normal input range.
After that the second “Slow adaptation” phase
occurs and lasts for about ten-fifteen seconds. Its
main task is to fine tune the input of the ganglion
cell and bring it completely to the middle point of
the ganglion cell’s dynamic range.
In case, when the contrast changes from high to
low (negative contrast change, e.g., going from sun
light to the room with normal light conditions), the
retina reacts differently. There is no fast adaptation
process, but the retina increases step-by-step the
sensitivity of the ganglion cells by scaling up their
inputs (with help of CAN). It takes up to twenty-
twenty five seconds till the inputs of the ganglion
cells are in their dynamic range.
These two statements were confirmed by
Solomon et al (Solomon et al., 2004) while
observing the reaction of the isolated retina of a tiger
salamander during contrast changes. Fig. 5 shows
the adaptation process for negative and positive
contrast changes.
A BIO-INSPIRED CONTRAST ADAPTATION MODEL AND ITS APPLICATION FOR AUTOMATIC LANE MARKS
DETECTION
515
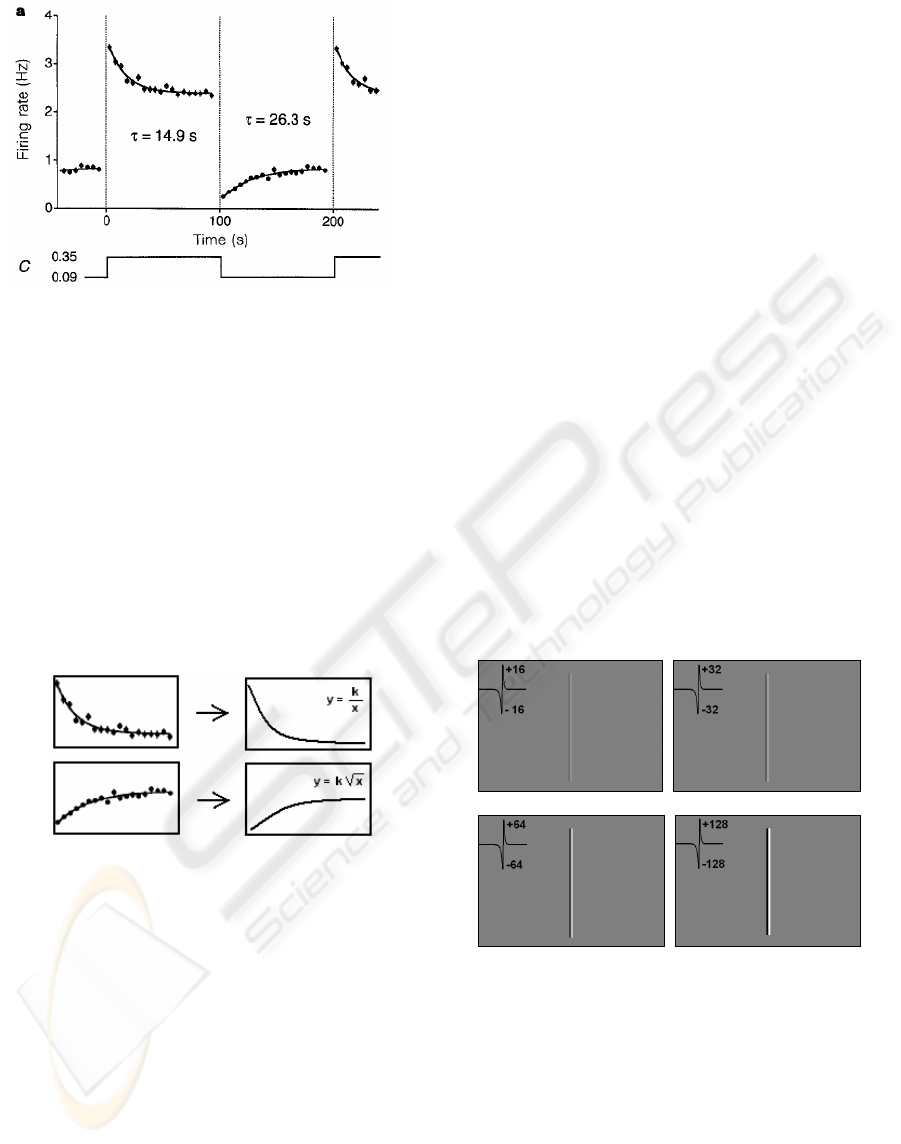
Figure 5: Contrast adaptation in salamander’s retina from
(Solomon et al., 2004).
In fig. 5 C depicts the contrast change values
while the graphical representation shows the
adaptation in the Salamanders retina on different
contrast changes.
We investigated which functions might
approximate the curves for “negative” and
”positive” adaptation and found out that for the
approximation of the “positive” contrast adaptation
process (fig. 6a, upper image) a simple rational
function (fig. 6b, upper image) can be used.
“Negative” contrast adaptation curve (fig. 6a, lower
image) can be approximated by the square root
function, which is shown in fig. 6b (lower image).
a) b)
Figure 6: a) Natural adaptation curves (from (Solomon et
al., 2004)) and b) their approximation functions.
Here, in both functions the coefficient k is a
scaling factor, which is responsible for the CAN’s
selectivity. It controls how strong the adaptation
should be in order to make the ganglion cells more
or less sensitive, depending on the current light
intensity situation. For instance, when the light
intensity is high (e.g., in sunny midday) than the
CAN should scale the intensity down by setting a
rather large k; however, when the light intensity is
just a bit above the dynamic range, the CAN should
fine tune the contrast by setting a quite small value
for the scaling coefficient. In this work we use the
fuzzy-like sets for the definition of CAN’s selectivity
coefficient k.
3 USING FUZZY-LIKE SETS FOR
CONTRAST ADAPTATION
In recent decades a number of applications were
found for fuzzy logic in economics, mathematics
and engineering. Firstly introduced in (Zadeh, 1965),
it is very helpful for modelling highly nonlinear
processes like natural contrast adaptation
3.1 Definition of a Fuzzy – like Set for
Normal Contrast
For the graphical representation of the model’s
response we should declare, what the Normal
contrast means and create a corresponding fuzzy –
like set for its definition.
Since we are working with bio-inspired edge
extraction based on zero-crossings, we assume here
that the absolute zero, as it shown on the
characteristic curve in fig. 2 will be equal to the
intensity 128, which represents the middle point of
the intensity spectrum. When model analyzes the
border between object and background, on the
graphical representation the response will drop down
and then raise up by a certain value (e.g., dark and
light vertical lines in fig. 3, image 2).
Then we analyzed which intensity differences
can represent the Normal Contrast value (see fig.7).
Figure 7: Biological edges with 16, 32, 64 and 128
intensity difference levels.
Fig. 7 shows four biological edges with
intensities 16, 32, 64 and 128. The edges with the
intensity differences of 16 and 32 do not have
enough contrast and should be adapted. The edges
with intensity difference of 64 and 128 do have
enough contrast and thus there is no need for
adaptation. However, in the real world situation the
biological intensity difference of 128 is hardly
possible, because it causes an intensity change of
255 levels at the object-background border (e.g.,
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
516
changing from black to white). Normally the
contrast numbers, which can be detected in real
images, lie in the range from 120 to 200, which
caused the biological edge of [± 60 – ±100] to
appear. That is why we do not have to adapt the high
intensity values (e.g., from 180 to 255), we should
only define such a process, which will adapt the
edge values from the lower part of the intensity
difference spectrum and bring them in to the middle
region. Thus, only the “negative” contrast adaptation
process should be used (fig. 6, lower images).
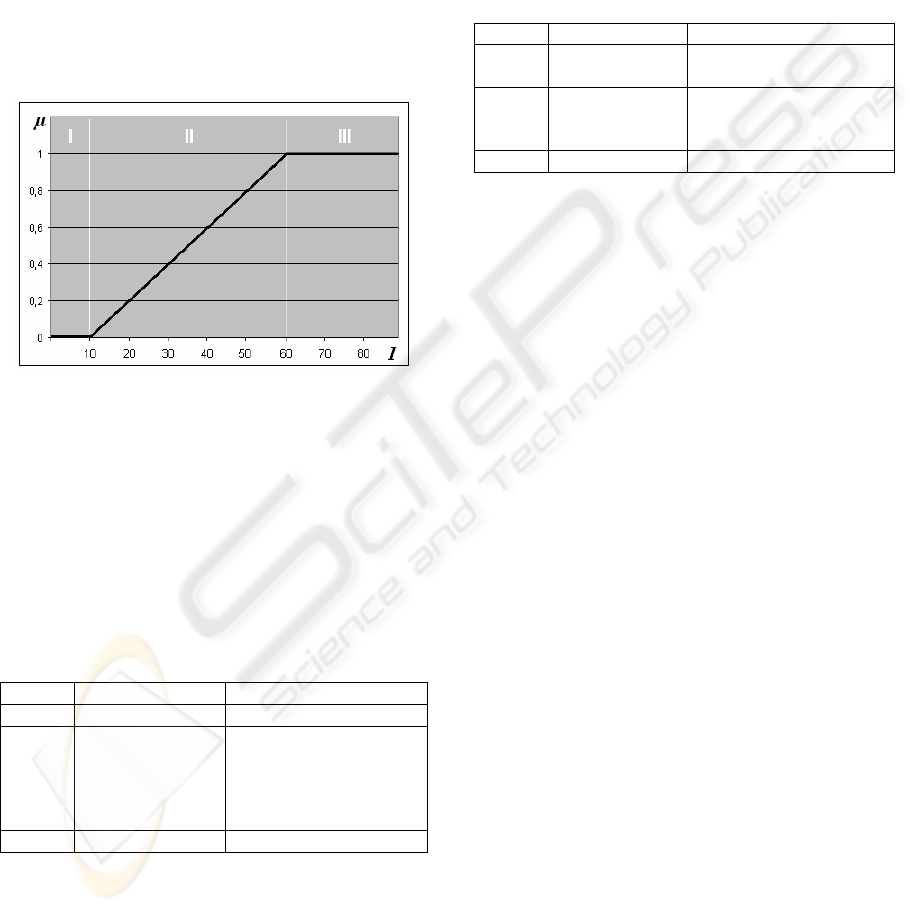
Following this, we introduce a Normal Contrast
fuzzy variable which should adapt all the values that
lie under the intensity 60. It is presented in fig. 8.
Figure 8: Fuzzy variable for Normal Contrast.
On this image, the X-axis represents the intensity
change I on the biological edge and Y-axis shows
the membership μ of a certain intensity value in the
Normal Contrast variable.
There are three characteristic adaptation regions
presented on this graphic. Since the fuzzy logic
operates with linguistic variables, table 1 shows such
a linguistic description and action which is needed
for a certain region.
Table 1: Linguistic definition of the model.
Region Intensity Action needed
I Low Intensity Strong adaptation
II Low–to–Normal
Intensity
Adaptation based on the
μ membership
coefficient in order to
control adaptation
strength
III Normal Intensity No adaptation needed
When the bipolar cells deliver low intensities
(values from 1 to 10), strong adaptation is needed; in
the mid-range (values from 11 and 60), adaptation is
also needed, but the system should control the
strength of the adaptation by using the membership
coefficient μ; and when the intensity is normal
(values above 61), then no adaptation is needed.
In order to create the system we should define
the set of rules for each of the regions
mathematically. Since we are using the “negative”
adaptation process, a curve that will represent this
process should have the shape of the square root
function. Table 2 shows the mathematical
representation for each action regions.
Table 2: Mathematical representation of the model.
Region Intensity values Representation
I 1 – 10 K = 2 ·√x
I
new
= I
i
· K
II 11 – 60 μ = (2 · I
i
+ 20) / 100
K = (2 - μ) · √x
I
new
= I
i
· K
III 61 – 127 I
new
= I
i
The adaptation process in nature lasted for
several seconds. Here this process is modelled with
iteration mechanism and x represents current
iteration; K is an adaptation coefficient and should
be calculated differently for regions I, II and III. It
represents the CAN selectivity and controls the input
gain to the ganglion cells. I
i
represents the input
intensity of CAN and I
new
is a new calculated value
of the adapted intensity; μ is a membership
coefficient, which influences the amplification factor
and is calculated according to the equation of the
characteristic line in region II (see fig. 8).
3.2 Adaptation Algorithm
The algorithm for the contrast adaptation involves
all the definition for the variables that have been set
early, like I
i
, I
low
, I
normal
, I
new
, μ, K and x which is
initially set to 0. Firstly, based on the current
intensity I
i
, μ is calculated.
if (I
i
>1&&I
i
<=I
low
){μ = 0}
if (I
i
>I
low
&&I
i
<=I
normal
){
μ = (2·I
i
+20)/100}
if (I
i
>I
normal
){μ = 1}
Then the adaptation coefficient K and the
adapted intensity I
new
based on the equations in table
2 is calculated.
if (μ<1){
while (I
new
<=I
normal
){
K = (2 - μ) · √x
I
new
= I
i
· K
x = x + 1;
}
}
A BIO-INSPIRED CONTRAST ADAPTATION MODEL AND ITS APPLICATION FOR AUTOMATIC LANE MARKS
DETECTION
517

else {I
new
=I
i
}
The process stops, when the calculated intensity
I
new
reaches the normal intensity I
normal
that has been
set to 60 empirically.
3.3 Adaptation Results
During the investigation and development the model
has been tested on different types of images.
Experiments were divided into three categories by
the specific adaptation process:
• adaptation of the low contrast;
• adaptation of the low-to-normal contrast;
• adaptation of the real world images;
The first two categories were tested with
synthetic images. Synthetic images were chosen
because the results of the processing can be
predicted in order to make the model’s proof of
concept under different conditions. To demonstrate
it a number of images with different intensity
changes were chosen. Fig. 9 represents two of them.
a) b)
Figure 9: Experimental data.
Fig. 9a shows the intensity change of 10 levels
(from 255 to 245) and figure 9b corresponds to a
change of 50 levels (from 255 to 205) of the
intensity spectrum. The digits on the images
represent just the absolute intensities and will not
appear in the modelling results.
Fig. 10 shows the calculated bipolar cells
activity for fig. 9a and 9b correspondently.
a) b)
Figure 10: Calculated bipolar cell’s responses to the
experimental data in fig. 9.
For the first experiment we took fig. 10a. The
initial data (fig. 9a) shows minor intensity change at
the object-background border, which causes a low
contrast and hardly distinguished border response
(fig. 10a). The initial intensity change I
i
equals 3
(see equations 1 and 2), which corresponds to the
low intensity region in fig. 8. Initial data: I
i
= 3; μ =
0, => K = 2 ·√x.
For the adaptation of such a low intensity 104
iterations are needed. Table 3 shows some of them.
Table 3: First experiment data.
x K I
new
Graphical representation
1 2 6
2 2.82 8
3 3.46 10
4 4 12
…
101 20.09 60
102 20.19 60
103 20.30 60
104 20.40 61
The second experiment has been performed with
fig. 10b. The initial intensity change here I
i
is 16,
which corresponds to the low-to-normal intensity
region in fig. 8. Adaptation is still needed, but the
strength of the adaptation should be controlled.
Initial data: I
i
= 16; μ = 0.12, => K = (2 - μ) · √x.
According to the algorithm, the adaptation of
such intensity will be done in 5 iteration steps. Table
4 represents this process.
Table 4: Third experiment data.
x K I
new
Graphical representation
1 1.88 30
2 2.65 42
3 3.25 52
4 3.76 60
5 4.20 67
x = 5, I
new
= 67:
As it can be seen on the results presented above,
the contrast adaptation model shows the expected
responses on the different stimuli with different
adaptation time. The intensity change adaptation
correlates with its natural representation (fig. 6,
lower image). To confirm this, fig. 11 shows the
adaptation curves for each experiment.
a) b)
Figure 11: Adaptation curves for all experiments.
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
518
3.4 Adaptation of the Real World
Images
The model has been already tested on the synthetic
images; the next step is to see how it will respond on
the real world images. For this purpose we choose a
number of images with the real road scenes that have
been taken on a german highway. Some of these test
images are shown in Fig. 12.
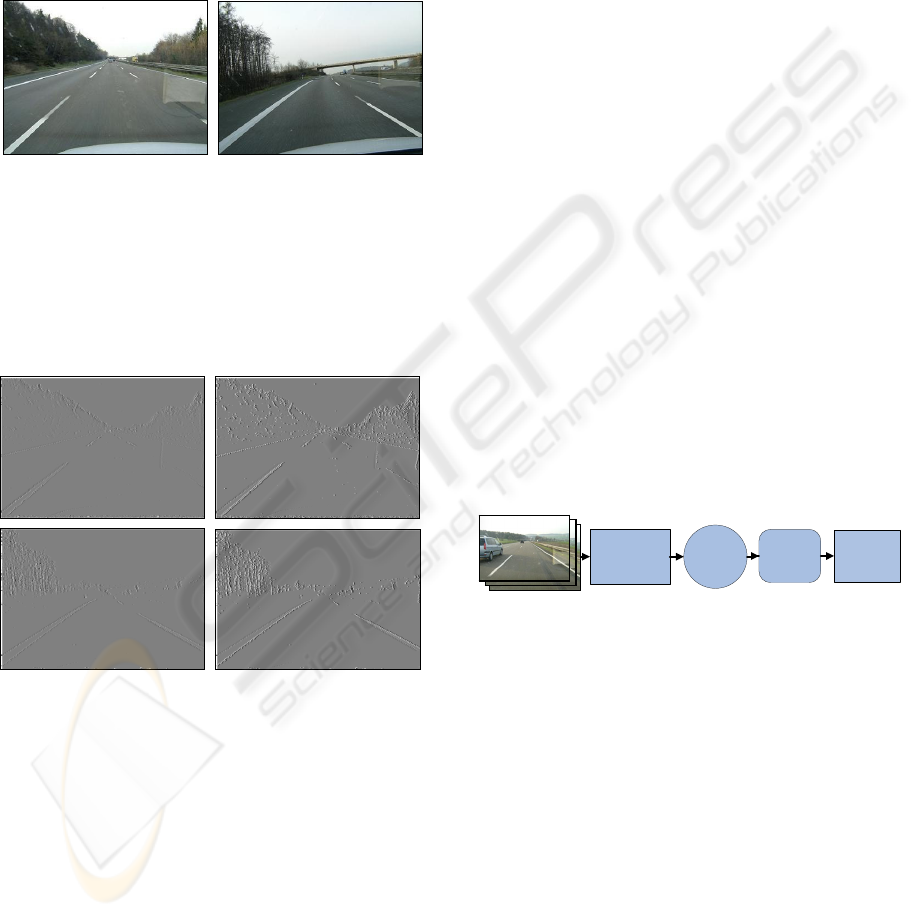
Figure 12: Real road scenes.
Then we processed the images first with the
classical biological edge operator without the
contrast adaptation mechanism. On the 2
nd
phase the
same images have been processed with the bio-
inspired edge operator and with the contrast
adaptation module. Fig. 13 shows the results.
Figure 13: Calculated biological edge without (left) and
with (right) contrast adaptation for fig. 12.
The difference between the adapted and the not
adapted images can be clearly seen. The edges, that
are even not fully visible on the left images, are well
seen on the right ones. Besides, the initial images
(fig. 12) were taken under slightly different
illumination conditions: the first image was taken
under bright sun light while the second one at early
evening. Nevertheless, the adapted images show
good results especially in underlining the lane road
marks. This gives the possibility to use this contrast
adaptation model for robust lane detection.
4 LANE DETECTION
APPLICATION
Lane keeping assistant systems have been described
in a number of recent publications, e.g. (Risack et
al., 2000), (Chang et al., 2003). For such systems
detection of the lane marks is a key feature for
further processing. The lane marks form lines with
certain slopes and thus, for its detection a good
shape extraction method is needed.
The Hough transformation (Leavers, 1992) is a
pattern recognition technique which is known for its
performance in locating given shapes in images.
Some researches have reported that the Hough
transform correlates with the processes that happen
in the striate cortex and in fact, reproduces the
natural mechanism of objects contour extraction
(Hubel, 1995), (Blasdel, 1992), (Ballard et al.,
1983), (Brueckmann et al., 2004).
Very interesting state-of-the-art research work is
presented in (Serre, 2007). The authors describe the
usage of the midbrain biological mechanisms for the
real world scene segmentation and objects
recognition. Furthermore, they also use the Hough
transformation as a shape localization method.
That is why we propose to use the Hough
transform as a lane marks detection method together
with the retina model with contrast adaptation as a
preprocessing method. This gives the possibility to
create a fully bio-inspired system for the lane mark
detection. The architecture of such a system is
shown in fig. 14.
Image
Acquisition
& Biological
Edge Detection
Hough
Processing
Contrast
Adaptation
Results
Interpretation
Road Image Sequences
Figure 14: Architecture of the bio-inspired lane detection
system.
The biological edge detection and contrast
adaptation stages were well described above. In fig.
14, after contrast adaptation the Hough
transformation takes place. Hough transformation
involves a voting scheme for the shape detection. In
particular, here we extract the lines of the different
slopes. Fig. 15 represents the Hough spaces built
from road edge picture (fig. 13, two right images)
and then the maximas are marked.
A BIO-INSPIRED CONTRAST ADAPTATION MODEL AND ITS APPLICATION FOR AUTOMATIC LANE MARKS
DETECTION
519

Figure 15: Hough spaces with local maximas.
After the maxima were detected, the
interpretation of the results should be performed.
Each maximum on the Hough space corresponds to
the line with a certain slope in a Cartesian space and
after processing the detected lane marks will be
highlighted. Fig. 16 shows the final results.
Figure 16: Detected lane marks in the adapted images.
5 CONCLUSIONS AND FUTURE
WORK
In this paper a bio-inspired model for contrast
adaptation has been presented. The model has been
tested with different test sets and showed good
results. Furthermore, the proposed contrast
adaptation algorithm has been coupled with the
Hough-based lane marks detector. This coupling
showed good performance and full correspondence
to the predicted behaviour.
Future work will concentrate on development of
the lane keeping assistant system using the bio-
inspired techniques further. In particular, for the
preprocessing stage the colour perception model will
be investigated, implemented and will be used for
the road scenes segmentation and traffic signs
detection.
Besides, for the post-processing and trajectory
prediction stages time-to-lane crossing approach will
be taken in to the account. It is likely possible that it
might be modelled with the natural timing delay-
computational maps. This problem will be also
investigated and the results will be reported.
REFERENCES
David H. Hubel, Eye, Brain and Vision, Freeman, 1995.
Linda G. Shapiro, George C. Stockman, Computer Vision,
Prentice-Hall , 2001.
Bence P. Ölveczky, Stephen A. Baccus & Markus Meister,
“Segregation of the object and background motion in
the retina,” Nature, Vol. 423, May 2003, pp. 401 –
408.
Richard H. Masland, “The fundamental plan of the
Retina,” Nature Neuroscience, Vol.4 No. 9, September
2001, pp. 877 – 886.
Michael J. Berry II, Iman H. Brivanlou, Thomas A. Jordan
& Markus Meister, “Anticipation of moving stimuli by
the retina,“ Nature, Vol. 398,March 1999, pp. 334–
338.
Hugh R. Wilson, “Nonlinear processes in the visual
discrimination”, Proc. Natl. Acad. Sci. USA, Vol. 90,
1993, pp. 9785 – 9790.
Stelios M. Smirnakis, Michael J. Berry, David K.
Warland, William Blalek & Markus Meister,
“Adaptation of retinal processing to image contrast
and spatial scale,” Nature, Vol. 386,March 1997,pp.
69–73
Stephan A. Baccus & Markus Meister, “Retina Versus
Cortex: Contrast Adaptation in Parallel Visual
Pathways”, Neuron, 2003, pp. 5 – 7.
Stephan A. Baccus & Markus Meister, “Fast and Slow
Contrast Adaptation in Retinal Circuitry,” Neuron,
Vol. 36, 2002, pp. 909 – 919.
Samuel Solomon, Jonathan Peirce, Neel Dhruv, Peter
Lennie, “Profound Contrast Adaptation Early in the
Visual Pathway”, Neuron, vol. 42, 2004, pp. 155–162
Zadeh L.A., “Fuzzy Sets”, Information and Control, 1965,
vol. 8, pp. 338 - 353.
Marr D., Vision, Freeman, pp. 54 – 78. 1982.
Risack, R.; Mohler, N.; Enkelmann, W., A video-based
lane keeping assistant, Intelligent Vehicles
Symposium, 2000. Proceedings of the IEEE, pp. 356 –
361, 2000.
Tang-Hsien Chang; Chun-Hung Lin; Chih-Sheng Hsu;
Yao-Jan Wu. A vision-based vehicle behavior
monitoring and warning system
Proceedings IEEE, Volume 1, pp. 448 – 453, 2003.
Leavers, V.F., Shape detection in Computer Vision using
Hough Transform. Springer – Verlag, p.201, 1992.
Gary G. Blasdel, “Orientation Selectivity, Preference, and
Continuity in Monkey Striate Cortex”, The Journal of
Neuroscience, Aug. 1992, 12 (8): 3139 – 3161.
Ballard D., Hinton J., Sejnowski T., Parallel visual
computation. Nature 306, pp. 21 – 26, 1983.
T. Serre, L. Wolf, S. Bileschi, M. Riesenhuber, and T.
Poggio, “Robust Object Recognition with Cortex-like
Mechanisms”, IEEE Trans. on Pattern Analysis and
Machine Intelligence, vol. 29, no. 3, March 2007.
Brueckmann, A., Klefenz F., Wuensche, A. A neural Net
for 2-D Slope and Sinusoidal Shape Detection.
International Scientific Journal of Computing. Vol. 3
(1), pp. 21 – 26, 2004.
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
520
